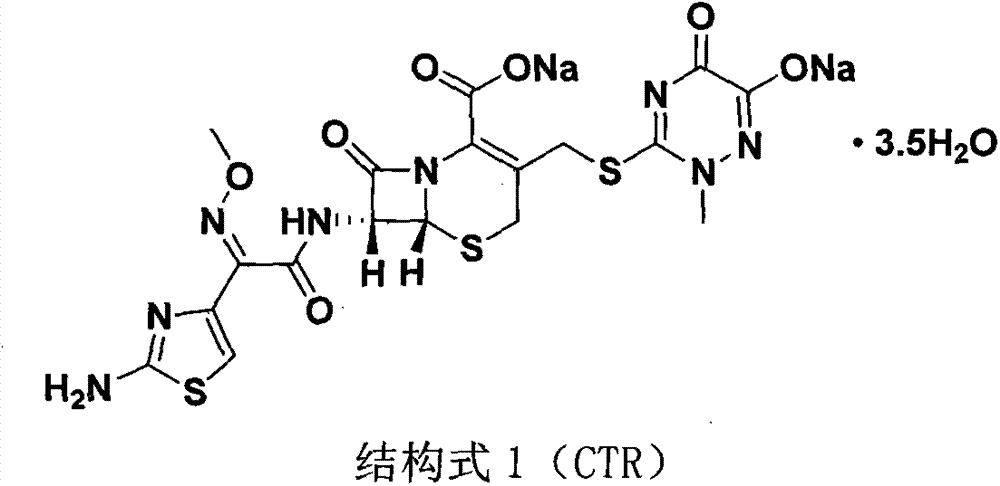
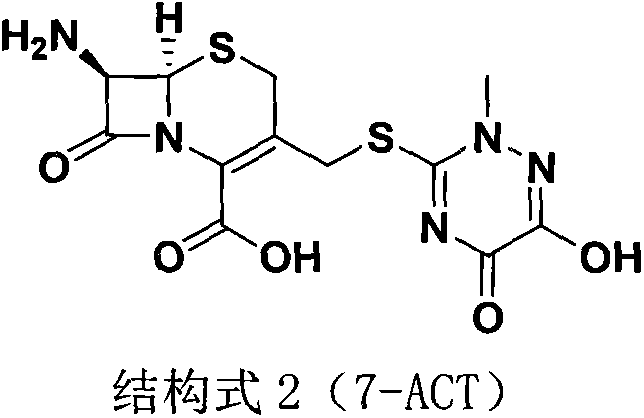
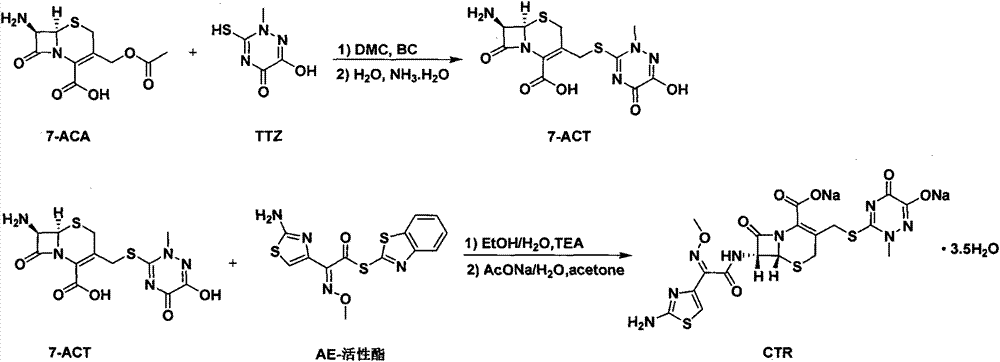
Method for preparing ceftriaxone sodium
A technology of ceftriaxone sodium and aminocephalosporanic acid, applied in the direction of organic chemistry, etc., can solve the problems of increasing the difficulty of solvent recovery, increasing the cost of producing ceftriaxone, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of 7-ACT
[0035] In a 1000ml three-necked flask, add in sequence: dimethyl carbonate (200ml), 7-ACA (35g, 128.5mmol), TTZ (20.5g, 128.8mmol), EDTANa 2 (0.4g, 1.2mmol) and boron trifluoride-dimethyl carbonate solution (188ml, 493.1mmol) (20% by mass), stirred and reacted for 40 minutes, and the temperature was controlled at T=30°C. After the reaction is completed, cool down to T3 ) hydrolysis to terminate the reaction, then dropwise add 5% ammonia water to seed the crystal until the system becomes turbid, and slowly stir to grow the crystal for 40 minutes. Then continue to drop 5% ammonia water to adjust the pH of the system to 3.8-4.0. After the adjustment is completed, slowly stir and grow the crystal for 30 minutes, and control the temperature T=6-7°C. Suction filtration, suction drying, the filter cake was washed with 40ml acetonitrile + 40ml water for the first wash, washed with 90ml acetone for the second wash, and dried in vacuum, and d...
Embodiment 2
[0036] Embodiment 2: Preparation of 7-ACT
[0037] In a 1000ml three-necked flask, add in sequence: dimethyl carbonate (200ml), 7-ACA (35g, 128.5mmol), TTZ (20.5g, 128.8mmol), EDTANa 2 (0.4g, 1.2mmol) and boron trifluoride-dimethyl carbonate solution (188ml, 493.1mmol) (20% by mass), stirred and reacted for 50 minutes, and the temperature was controlled at T=30°C. After the reaction is completed, cool down to T3 ) hydrolysis to terminate the reaction, then dropwise add 5% ammonia water to seed the crystal until the system becomes turbid, and slowly stir to grow the crystal for 40min. Then continue to drop 5% ammonia water to adjust the pH of the system to 3.8-4.0. After the adjustment is completed, slowly stir and grow the crystal for 30 minutes, and control the temperature T=6-7°C. Suction filtration, suction drying, the filter cake was washed with 40ml of acetonitrile + 40ml of water for the first wash, washed with 90ml of acetone for the second wash, dried in vacuum, and d...
Embodiment 3
[0038] Embodiment 3: Preparation of 7-ACT
[0039] In a 1000ml three-necked flask, add in sequence: dimethyl carbonate (200ml), 7-ACA (35g, 128.5mmol), TTZ (20.5g, 128.8mmol), EDTANa 2 (0.4g, 1.2mmol) and boron trifluoride-dimethyl carbonate solution (188ml, 493.1mmol) (20% by mass), stirred and reacted for 60 minutes, and the temperature was controlled at T=30°C. After the reaction is completed, cool down to T3 ) hydrolysis to terminate the reaction, then dropwise add 5% ammonia water to seed the crystal until the system becomes turbid, and slowly stir to grow the crystal for 40 minutes. Then continue to drop 5% ammonia water to adjust the pH of the system to 3.8-4.0. After the adjustment is completed, slowly stir and grow the crystal for 30 minutes, and control the temperature T=6-7°C. Suction filtration and suction drying, the filter cake was washed with 40ml of acetonitrile + 40ml of water for the first wash, washed with 90ml of acetone for the second wash, and then dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com