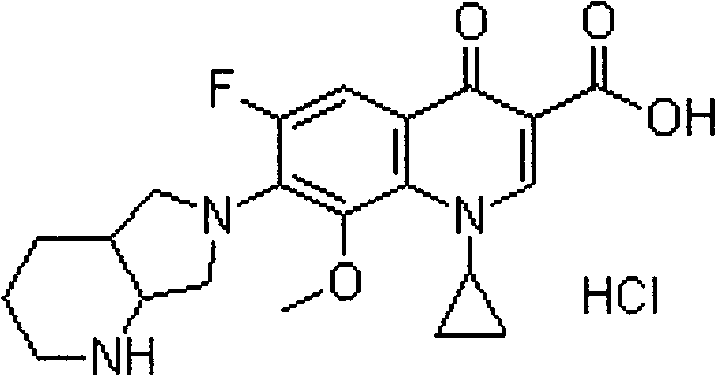
Stable moxifloxacin hydrochloride injection
A technology of moxifloxacin hydrochloride and moxifloxacin, which can be applied to medical preparations containing active ingredients, respiratory diseases, organic active ingredients, etc., can solve the problems of further improvement of stability, destruction of physiological environment, adverse reactions and the like, Achieve the effect of being conducive to large-scale industrial production, low industrialization cost, and reducing toxic and side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Moxifloxacin hydrochloride injection, containing 1.6mg / ml moxifloxacin hydrochloride and 42mg / ml xylitol.
[0045]
[0046] Take moxifloxacin hydrochloride and xylitol of the prescribed amount in the container, add an appropriate amount of water for injection and stir to dissolve it; adjust the pH to 4.31 with hydrochloric acid or sodium hydroxide solution, add water for injection to 6000ml; add 0.5% (g / v) Activated carbon, stirred for 30 minutes; first decarbonized by 0.45 μm filter, and then finely filtered by 0.22 μm to obtain the filtrate; respectively poured the filtrate into the packaging for injection, sealed; sterilized at 121° C. for 15 minutes to obtain moxifloxacin hydrochloride injection finished product.
Embodiment 2
[0048] Moxifloxacin hydrochloride injection, containing 0.4mg / ml moxifloxacin hydrochloride and 10mg / ml xylitol.
[0049]
[0050] Take moxifloxacin hydrochloride and xylitol of prescription quantity in container, add appropriate amount of water for injection and stir to make it dissolve; Regulate pH to 4.52 with hydrochloric acid or sodium hydroxide solution, add water for injection to 6000ml; Add 0.5% (g / v) Activated carbon, stirred for 30 minutes; first decarbonized by 0.45 μm filter, and then finely filtered by 0.22 μm to obtain the filtrate; respectively poured the filtrate into the packaging for injection, sealed; sterilized at 121° C. for 15 minutes to obtain moxifloxacin hydrochloride injection finished product.
Embodiment 3
[0052] Moxifloxacin hydrochloride injection, containing moxifloxacin hydrochloride 8mg / ml, xylitol 200mg / ml.
[0053]
[0054] Take moxifloxacin hydrochloride and xylitol of the prescribed amount in a container, add an appropriate amount of water for injection and stir to dissolve it; adjust the pH to 4.37 with hydrochloric acid or sodium hydroxide solution, add water for injection to 6000ml; add 0.5% (g / v) Activated carbon, stirred for 30 minutes; first decarbonized by 0.45 μm filter, and then finely filtered by 0.22 μm to obtain the filtrate; respectively poured the filtrate into the packaging for injection, sealed; sterilized at 121° C. for 15 minutes to obtain moxifloxacin hydrochloride injection finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com