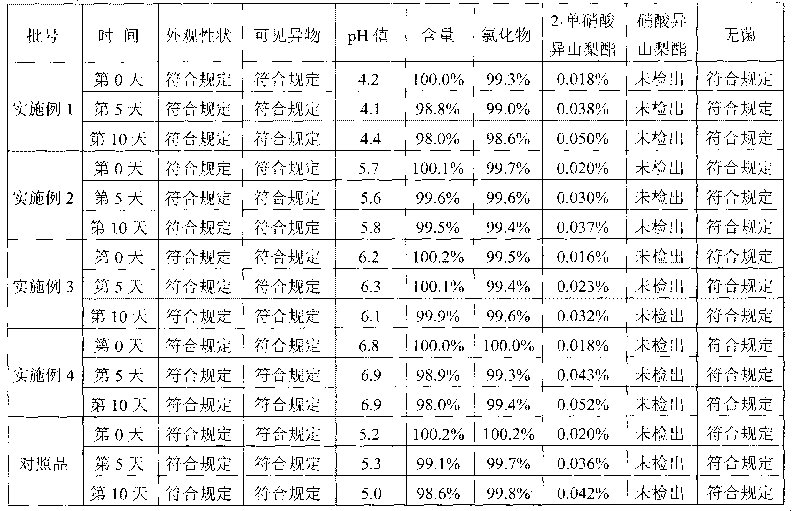
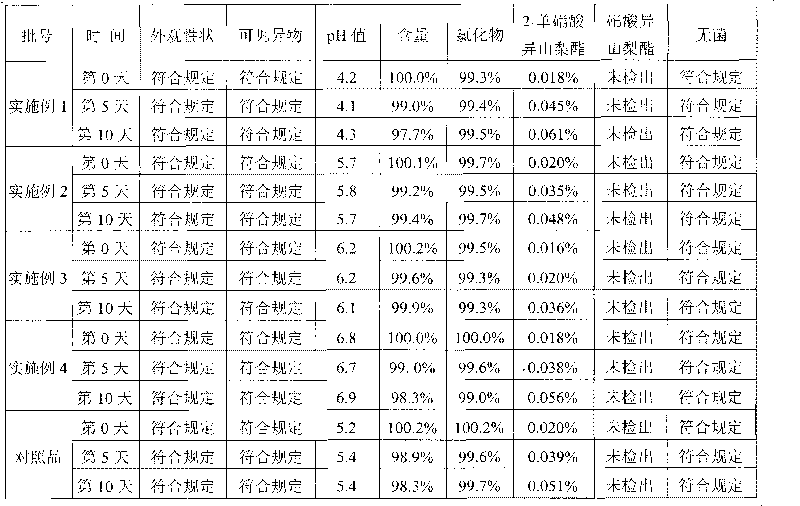
Isosorbide mononitrate sodium chloride injection
A technology of isosorbide dinitrate and sodium chloride injection, applied in the direction of active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, cardiovascular system diseases, etc., can solve the problem of reduced curative effect, low bioavailability, and uncomfortable patients and other issues to achieve the effect of ensuring product safety, simple treatment of three wastes, and avoiding secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Isosorbide Mononitrate 10mg
[0028] Sodium chloride 450mg
[0029] 0.05mol / L sodium hydroxide solution appropriate amount
[0030] Add water for injection to 100ml
[0031] Preparation process: first prepare the concentrated sodium chloride solution and filter it, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH of the liquid to 4.3 with an appropriate amount of 0.05mol / L sodium hydroxide solution, and fill it out Bacteria, that is.
Embodiment 2
[0033] Isosorbide Mononitrate 20mg
[0034] Sodium chloride 900mg
[0035] 0.05mol / L sodium hydroxide solution appropriate amount
[0036] Add water for injection to 100ml
[0037] Preparation process: first prepare the concentrated sodium chloride solution and filter it, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH value of the liquid to 5.7 with an appropriate amount of 0.05mol / L sodium hydroxide solution, and fill it out Bacteria, that is.
Embodiment 3
[0039] Isosorbide Mononitrate 20mg
[0040] Sodium chloride 900mg
[0041] 0.1mol / L sodium hydroxide solution appropriate amount
[0042] Add water for injection to 100ml
[0043] Preparation process: First prepare the concentrated sodium chloride solution and filter it, add isosorbide mononitrate and stir to dissolve, add water for injection to the full amount, adjust the pH of the liquid to 6.2 with an appropriate amount of 0.1mol / L sodium hydroxide solution, and fill it out Bacteria, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com