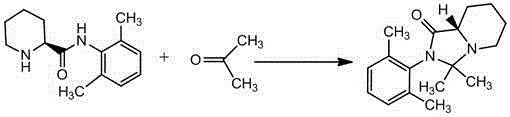
A kind of preparation method of ropivacaine hydrochloride impurity f
A technology for ropivacaine hydrochloride and impurities, which is applied in the field of chemical synthesis, can solve the problems such as the synthetic method of impurity F that is not reported in public data, and achieves the effects of high product purity, short synthesis route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 100g of acetone and 20g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide were added to a 250ml clean three-necked flask equipped with mechanical stirring, and the solids were completely dissolved by stirring and heating. Then add 5 g of acetic acid, continue to heat up to reflux, and after 5 hours of reaction, the thin-layer detection of the raw materials basically completes the reaction (TLC conditions: dichloromethane: methanol = 10:1). The solvent was slowly evaporated under normal pressure, and after the solvent distillation was completed, 300 g of deionized water at 50° C. was added to the residue, and a solid was slowly precipitated, and the pH value was adjusted to 9 with sodium hydroxide solution. After filtration, the filter cake was washed with deionized water and dried to obtain 19 g of crude product with a liquid phase purity greater than 97%. 19g of crude product was added to 76g of ethanol, heated to reflux for 30 minutes to dissolve the solid; cooled to roo...
Embodiment 2
[0024] 200g of acetone and 25g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide were added to a 500ml clean three-necked flask equipped with mechanical stirring, and the solids were completely dissolved by stirring and heating. Then add concentrated hydrochloric acid 15g, continue to heat up to reflux, after 10 hours of reaction, the thin layer detects that the raw material has basically reacted (TLC conditions methylene chloride: methanol=10:1). The solvent was slowly evaporated under normal pressure. After the solvent was distilled, 375 g of deionized water at 50° C. was added to the residue, and the solid was slowly precipitated, and the pH was adjusted to 9 with sodium hydroxide solution. After filtration, the filter cake was washed with deionized water and dried to obtain 24 g of crude product with a liquid phase purity greater than 96%. 24g of crude product was added to 168g of methyl isobutyl ketone, heated to reflux for 30 minutes to dissolve the solid; cooled to room te...
Embodiment 3
[0026] 75g of acetone and 25g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide were added to a 250ml clean three-necked flask equipped with mechanical stirring, and the solid was dissolved by stirring and heating. Then add sulfuric acid 2.5g, continue to heat up to reflux, after 8 hours of reaction, the thin layer detects that the raw material is basically reacted (TLC conditions methylene chloride: methanol=10:1). The solvent was slowly evaporated under normal pressure, and after the solvent distillation was completed, 375 g of deionized water at 50°C was added to the residue, and a solid was slowly precipitated, and the pH value was adjusted to 9 with sodium hydroxide solution. Filtration, the filter cake was washed with deionized water, and dried to obtain 25 g of crude product with a liquid phase purity greater than 96%. 25g of crude product was added to 50g of acetonitrile, heated to reflux for 30 minutes to dissolve the solid; cooled to room temperature, filtered, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com