Ropivacaine hydrochloride injection and preparation method thereof
A technology for ropivacaine hydrochloride and injection, which is applied in the field of drug preparation, and can solve the problems of high oxygen permeability rate, dextroisomer and other related impurities produced by ropivacaine hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
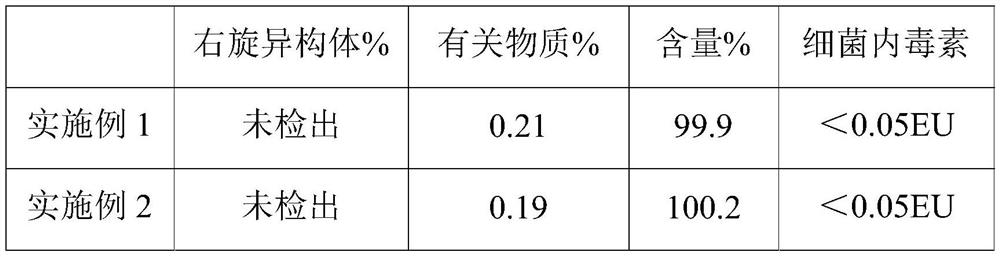
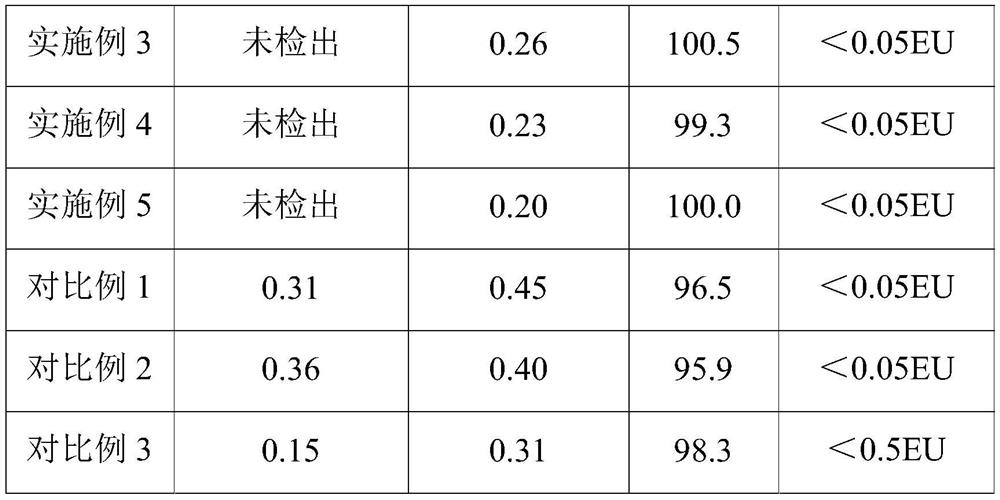
Examples
Embodiment 1
[0025] The present embodiment provides a kind of preparation method of ropivacaine hydrochloride injection, and specific preparation steps are as follows:
[0026] Dissolve 100kg of ropivacaine hydrochloride and 71kg of sodium chloride in 10L of water for injection, then adjust the pH value to 5.6 with 0.01mol / L sodium hydroxide solution to obtain a medicinal solution;
[0027] Pass the drug solution through polyethersulfone folded filter elements with pore diameters of 0.2 μm and 0.1 μm at 20° C. to obtain a filtrate for use;
[0028] Under the protection of A-level laminar flow, the three-in-one method of blow-fill-seal is used to blow molding in turn to form empty polypropylene ampoules, which are filled with N 2 Fill the above-mentioned filtrate, seal, sterilize at 121°C for 10 minutes, and use high-voltage discharge to detect leaks after cooling down to room temperature, to obtain the ropivacaine hydrochloride injection;
[0029] Among them, in the step of blow molding t...
Embodiment 2
[0032] The present embodiment provides a kind of preparation method of ropivacaine hydrochloride injection, and specific preparation steps are as follows:
[0033] Dissolve 100kg of ropivacaine hydrochloride and 71kg of sodium chloride in 10L of water for injection, then adjust the pH value to 5.0 with 0.03mol / L sodium hydroxide solution to obtain a medicinal solution;
[0034] Pass the drug solution through polyethersulfone folded filter elements with pore diameters of 0.2 μm and 0.1 μm at 35° C. to obtain a filtrate for use;
[0035] Under the protection of A-level laminar flow, the three-in-one method of blow-fill-seal is used to blow molding in turn to form empty polypropylene ampoules, which are filled with N 2 Fill the above-mentioned filtrate, seal, sterilize at 121°C for 15 minutes, and use high-voltage discharge to detect leaks after cooling down to room temperature, to obtain the ropivacaine hydrochloride injection;
[0036] Among them, in the step of blow molding t...
Embodiment 3
[0039] The present embodiment provides a kind of preparation method of ropivacaine hydrochloride injection, and specific preparation steps are as follows:
[0040] Dissolve 100kg of ropivacaine hydrochloride and 71kg of sodium chloride in 10L of water for injection, then adjust the pH value to 4.2 with 0.02mol / L sodium hydroxide solution to obtain a medicinal solution;
[0041] Pass the drug solution through polyethersulfone folded filter elements with pore sizes of 0.2 μm and 0.1 μm at 28° C. to obtain a filtrate for use;
[0042] Under the protection of A-level laminar flow, the three-in-one method of blow-fill-seal is used to blow molding in turn to form empty polypropylene ampoules, which are filled with N 2 Fill the above-mentioned filtrate, seal, sterilize at 121°C for 20 minutes, and use high-voltage discharge to detect leaks after cooling down to room temperature to obtain the ropivacaine hydrochloride injection;
[0043] Among them, in the step of blow molding to for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com