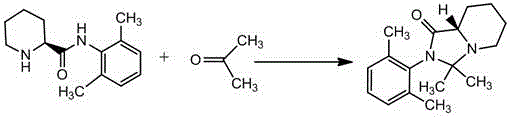
Preparation method of ropivacaine hydrochloride impurity F
A technology for ropivacaine hydrochloride and impurities, which is applied in the field of chemical synthesis, can solve the problems such as the synthetic method of impurity F that is not reported in public materials, and achieves the effects of high product purity, short synthesis route, and solving the difficult problem of quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 100 g of acetone and 20 g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide into a 250ml clean three-neck flask equipped with mechanical stirring, stir and heat to dissolve all the solids. Then 5 g of acetic acid was added, and the temperature continued to rise to reflux. After 5 hours of reaction, the raw materials were basically reacted by TLC (TLC conditions: dichloromethane: methanol = 10:1). Start to slowly evaporate the solvent under normal pressure. After the solvent distillation, add 300 g of deionized water at 50°C to the residue to slowly precipitate solids, and then adjust the pH to 9 with sodium hydroxide solution. After filtering, the filter cake was washed with deionized water and dried to obtain 19 g of crude product with a liquid phase purity greater than 97%. Add 19g of the crude product to 76g of ethanol, heat and reflux for 30 minutes to dissolve the solid; cool to room temperature, filter, and dry the filter cake to obtain 15.3g of impurity F pure p...
Embodiment 2
[0024] Add 200g of acetone and 25g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide into a 500ml clean three-neck flask equipped with mechanical stirring, stir and heat to dissolve all the solids. Then 15 g of concentrated hydrochloric acid was added, and the temperature was continued to reflux. After 10 hours of reaction, the raw materials were basically reacted by TLC (TLC conditions: dichloromethane: methanol = 10:1). Start to slowly evaporate the solvent under normal pressure. After the solvent distillation is completed, add 375 g of deionized water at 50°C to the residue to slowly precipitate a solid, and then adjust the pH value to 9 with sodium hydroxide solution. Filter, wash the filter cake with deionized water, and dry to obtain 24 g of crude product, the liquid phase purity is greater than 96%. Add 24g of the crude product to 168g of methyl isobutyl ketone, heat and reflux for 30 minutes to dissolve the solid; cool to room temperature, filter, and dry the filter cake ...
Embodiment 3
[0026] Add 75g of acetone and 25g of (S)-N-(2',6'-xylyl)-2-piperidinecarboxamide into a 250ml clean three-neck flask equipped with mechanical stirring, stir and heat to dissolve all the solids. Then add 2.5 g of sulfuric acid, continue to heat up to reflux, and react for 8 hours, and the raw materials are basically reacted by TLC (TLC conditions: dichloromethane: methanol = 10:1). Start to slowly evaporate the solvent under normal pressure, add 375 g of deionized water at 50°C to the residue after solvent distillation, and slowly precipitate solids, then adjust the pH value to 9 with sodium hydroxide solution. Filter, wash the filter cake with deionized water, and dry to obtain 25 g of crude product, the liquid phase purity is greater than 96%. Add 25g of the crude product into 50g of acetonitrile, heat and reflux for 30 minutes to dissolve the solid; cool to room temperature, filter, and dry the filter cake to obtain 19.4g of impurity F pure product; the liquid phase purity i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com