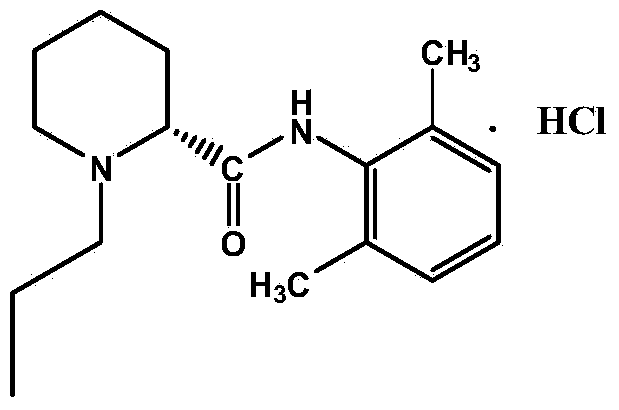
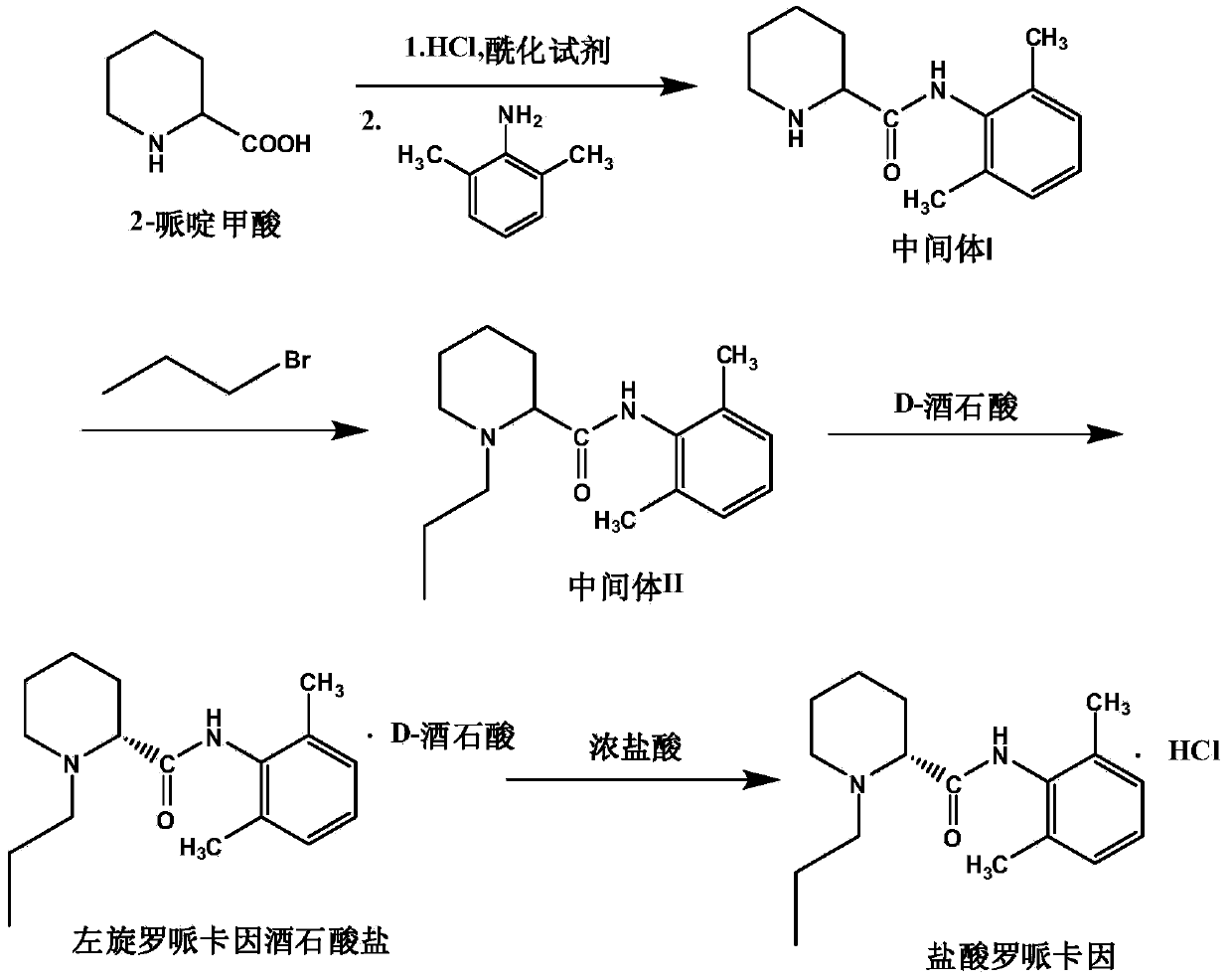
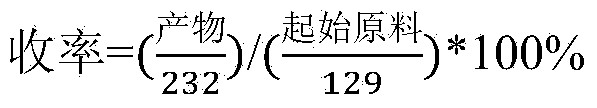Method for preparing hydrochloric acid ropivacaine
A technology for ropivacaine hydrochloride and lev-ropivaca is applied in the field of chemistry and can solve the problems of high cost of raw materials, unsuitability for industrial production, racemization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0032] Experimental example 1, the selection of intermediate (I) separation pH (aqueous phase)
[0033] One, the purpose of the experiment: in the process of investigating the preparation of intermediate (I), 2-piperidinecarboxylic acid and 2,6-dimethylaniline react in toluene solution to generate the hydrochloride of intermediate (I) and then reduce it to intermediate ( After I), the impact of different pH values on the yield of intermediate (I).
[0034]2. Method: 4 experiments in parallel——add 10.0g of 2-piperidinecarboxylic acid and 160ml of toluene into a 500ml reaction flask, feed HCl gas to pH 2-3, raise the temperature to 45-50°C, add 1.5ml of DMF (N,N -dimethylformamide), dropwise add 9.3g (1.0 equivalent) of the mixed solution of thionyl chloride and 20ml toluene, dropwise, keep warm at 50-55°C for 3h, add dropwise 37.5g, that is, dropwise add 4.0 equivalent of 2, Mixture of 6-dimethylaniline and 20ml of toluene, keep warm at 55-60°C for 3 hours, filter to obtain ...
experiment example 2
[0043] Experimental example 2, the selection of intermediate (I) separation extraction solvent
[0044] 1. Experimental purpose: To investigate the influence of different extraction reagents on the yield of intermediate (I) during the preparation of intermediate (I).
[0045] 2. Method: Four experiments in parallel——add 10.0g of 2-piperidinecarboxylic acid and 160ml of toluene into a 500ml reaction bottle, feed HCl gas to about pH2-3, raise the temperature to 45-50°C, add 1.5ml of DMF (N,N -Dimethylformamide), add dropwise 9.3g (1.0 equivalent) of the mixed solution of thionyl chloride and 20ml of toluene, dropwise, keep warm at 50-55°C for 3h, add dropwise 37.5g (ie 4.0 equivalent) of 2,6 - Mixture of dimethylaniline and 20ml of toluene, keep warm at 55-60°C for 3 hours, filter to obtain 65g of yellow-green wet product, dry to obtain 56g of gray solid, add the solid to 280ml of purified water, and stir to dissolve to obtain a reaction solution; Slowly add % NaOH solution to ...
experiment example 3
[0053] Experimental example 3, selection of catalyst in the resolution reagent in the preparation process of levopivacaine tartrate
[0054] 1. Purpose of the experiment: To investigate the separation results of different catalysts in the resolution reagent during the preparation of L-ropivacaine tartrate.
[0055] 2. Method: Four experiments in parallel—dissolve 14.8g of intermediate (I) in 60mlDMF, add 8.5gK 2 CO 3 , add 7.8g (1.0 equivalent) bromo-n-propane dropwise, after dropping, raise the temperature to 75-80°C, keep it warm for 2h; cool down to room temperature, filter, add the filtrate to 150ml ice water, precipitate a large amount of white solid, filter and dry , to obtain about 16.6g of white solid, yield 95%, which is intermediate (II) N-(2,6-dimethylphenyl)-1-n-propylpiperidine-2-carboxamide; 15g intermediate Compound (II) was dissolved in 100ml of isopropanol, heated to 40°C and stirred to dissolve; when completely dissolved, 0.1 equivalent of different catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com