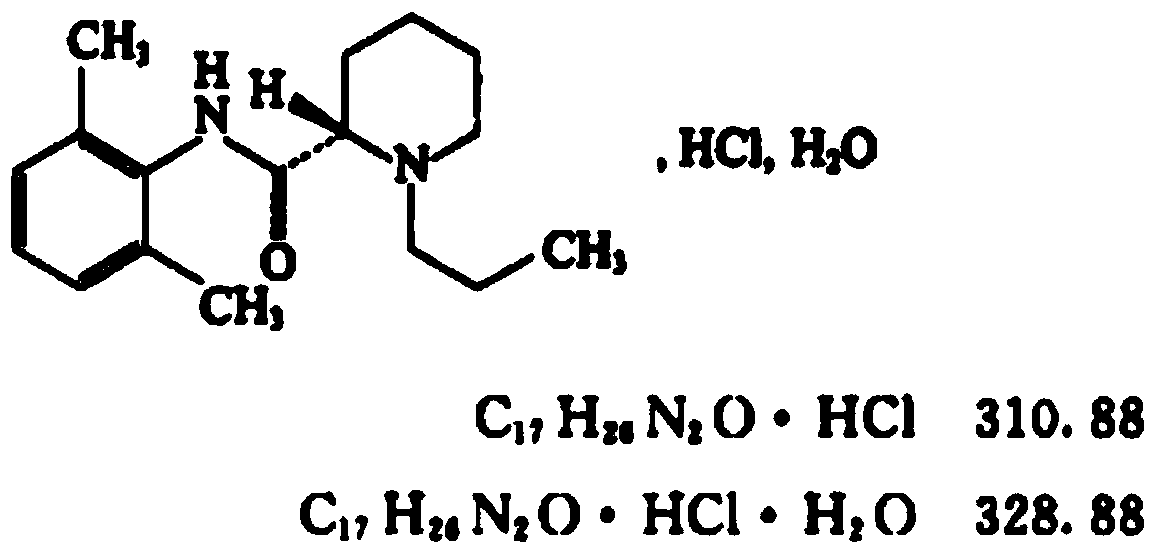
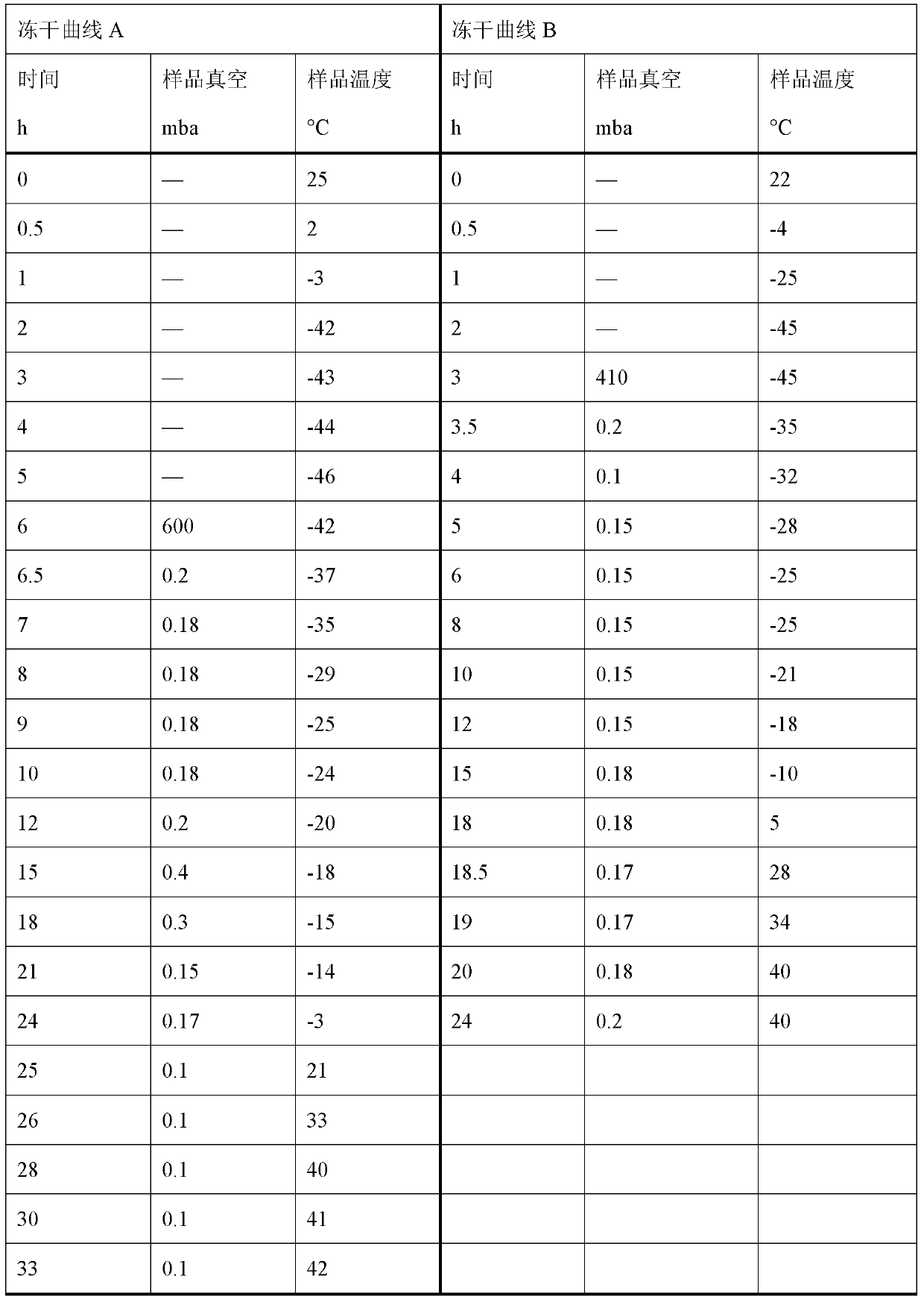
Freeze-dried ropivacaine hydrochloride composition for injection and its quality control method
A kind of technology of ropivacaine hydrochloride and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] Embodiment 1: Preparation of ropivacaine hydrochloride freeze-dried powder injection
[0130] formula:
[0131] Ropivacaine hydrochloride 75mg,
[0132] Lactose 40mg,
[0133] Acid-base regulator: appropriate amount, adjust the pH to the specified value in the following preparation method,
[0134] Water for injection: add to 3ml.
[0135] Preparation method:
[0136] (a) take by weighing ropivacaine hydrochloride, lactose of prescription quantity, add prescription quantity 60% water for injection, stir and make to dissolve;
[0137] (b) Add activated carbon (0.1% by volume of the medicinal liquid) to the medicinal liquid obtained in the previous step, adjust the pH value of the solution to 3.2 with an acid-base regulator, stir for 2.0 hours at a temperature of 25° C., filter for decarbonization, and use an acid-base regulator to The regulator adjusts the pH of the solution to 5.0;
[0138] (c) Add water for injection to the full amount of the prescription, ...
Embodiment 2
[0141] Embodiment 2: Preparation of ropivacaine hydrochloride freeze-dried powder injection
[0142] formula:
[0143] Ropivacaine hydrochloride 75mg,
[0144] Lactose 45mg,
[0145] Acid-base regulator: appropriate amount, adjust the pH to the specified value in the following preparation method,
[0146] Water for injection: add to 3.75ml.
[0147] Preparation method:
[0148] (a) take by weighing ropivacaine hydrochloride, lactose of prescription quantity, add prescription quantity 65% water for injection, stir and make to dissolve;
[0149] (b) Add activated carbon (0.05% by volume of the medicinal solution) to the medicinal solution obtained in the previous step, adjust the pH value of the solution to 3.0 with an acid-base regulator, stir for 2.0 hours at a temperature of 20°C, filter for decarbonization, and use an acid-base regulator to The regulator adjusts the pH of the solution to 5.5;
[0150] (c) Add water for injection to the full amount of the prescr...
Embodiment 3
[0153] Embodiment 3: Preparation of ropivacaine hydrochloride freeze-dried powder injection
[0154] formula:
[0155] Ropivacaine hydrochloride 75mg,
[0156] Lactose 35mg,
[0157] Acid-base regulator: appropriate amount, adjust the pH to the specified value in the following preparation method,
[0158] Water for injection: add to 2.5ml.
[0159] Preparation method:
[0160] (a) take by weighing ropivacaine hydrochloride, lactose of prescription quantity, add prescription quantity 55% water for injection, stir and make dissolving;
[0161] (b) Add activated carbon (0.15% by volume of the medicinal solution) to the medicinal solution obtained in the previous step, adjust the pH value of the solution to 3.5 with an acid-base regulator, stir for 2.3 hours at a temperature of 30° C., filter for decarbonization, and use an acid-base regulator to The regulator adjusts the pH4.5 of the solution;
[0162] (c) Add water for injection to the full amount of the prescription...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com