Ropivacaine hydrochloride and sodium chloride injection and preparation method
A technology of ropivacaine hydrochloride sodium chloride and ropivacaine hydrochloride, which is applied in the field of medicine, can solve the problems of difficulty in finding, introducing impurities, and hazards in the human body, and achieves high safety in use, stable product quality, and mature preparation technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
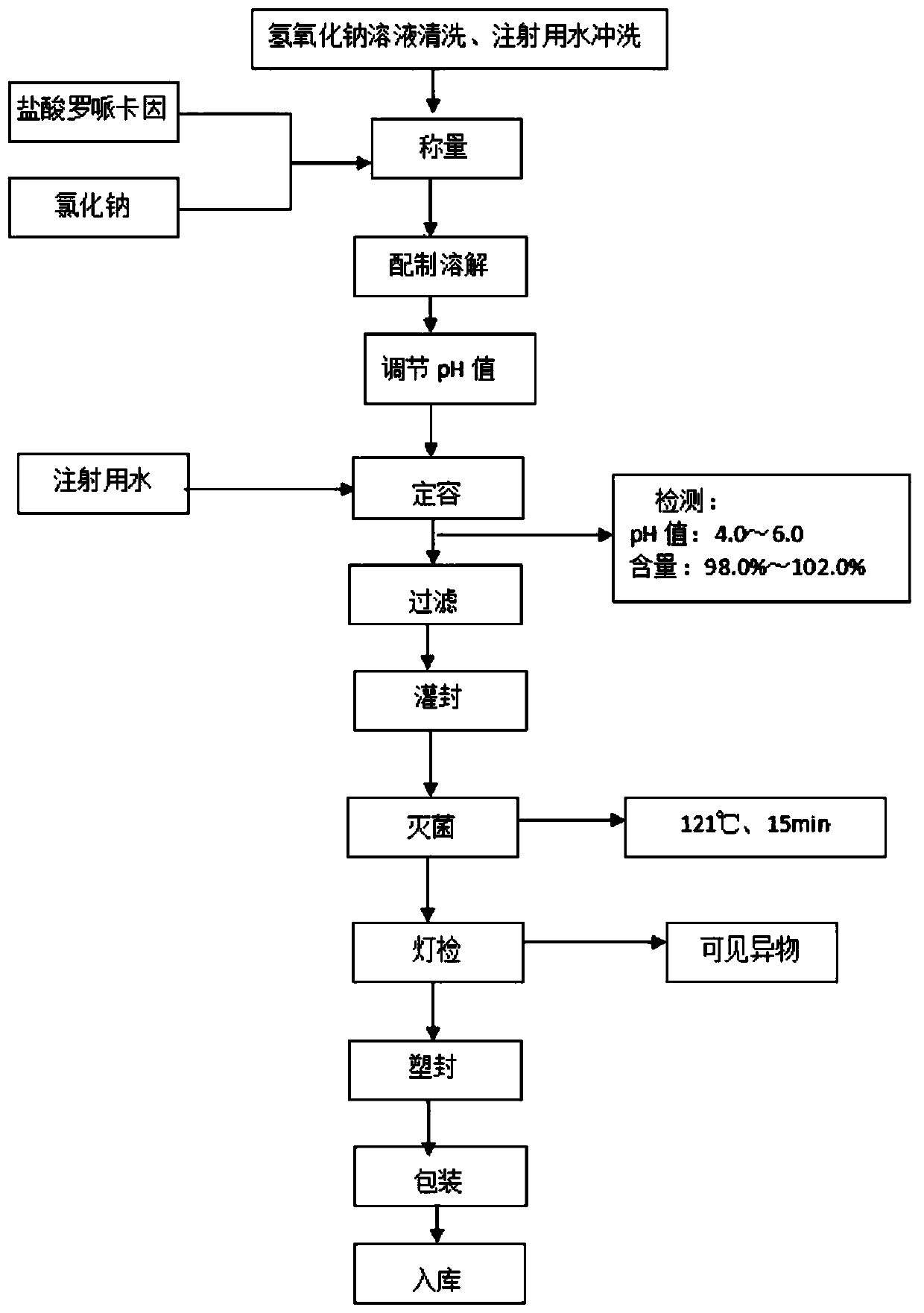
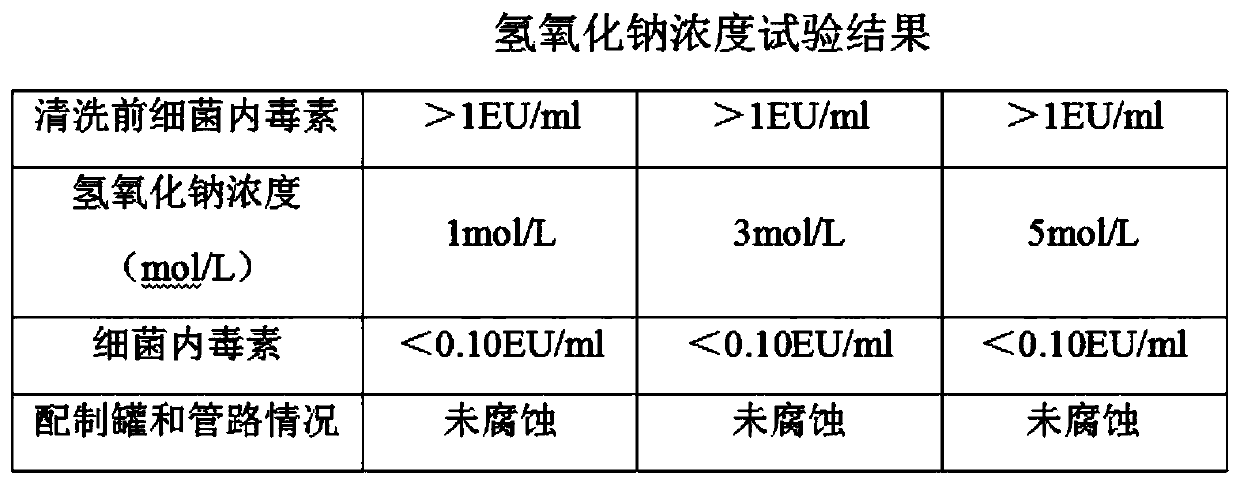
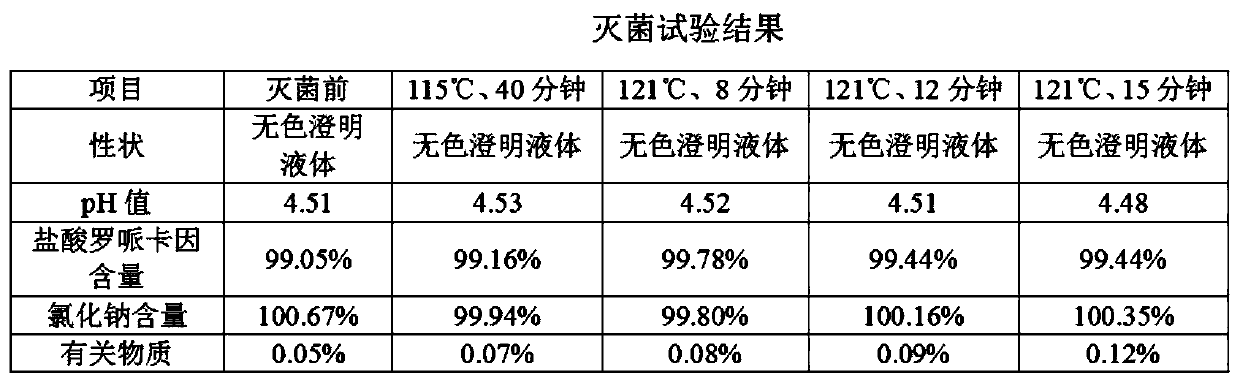
[0033] Preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings so that those embodiments can be easily realized by those having ordinary skill in the art to which the invention pertains. However, the present invention can also be realized in various forms, so the present invention is not limited to the embodiments described hereinafter. In addition, in order to describe the present invention more clearly, parts not connected with the present invention will be omitted from the drawings.
[0034] The embodiment of the present application provides a kind of ropivacaine hydrochloride sodium chloride injection, comprising: ropivacaine hydrochloride, sodium chloride and water for injection; taking 1L injection as an example, its prescription contains ropivacaine hydrochloride 1.0 -3.0g, sodium chloride 7.0-10.0g.
[0035] The embodiment of the present application provides a kind of ropivacaine hydrochloride sodium chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com