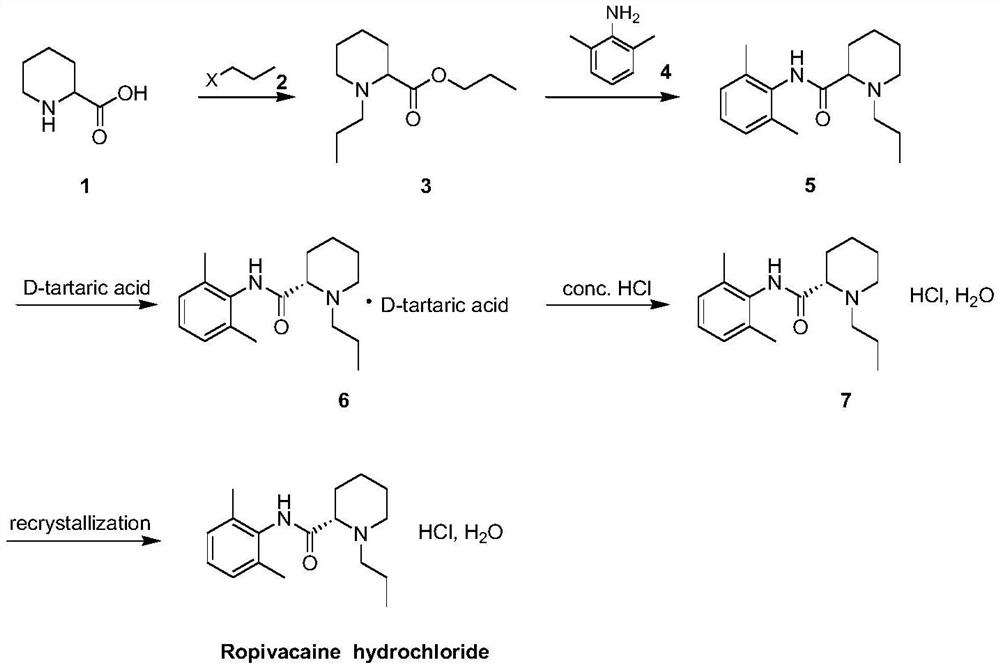
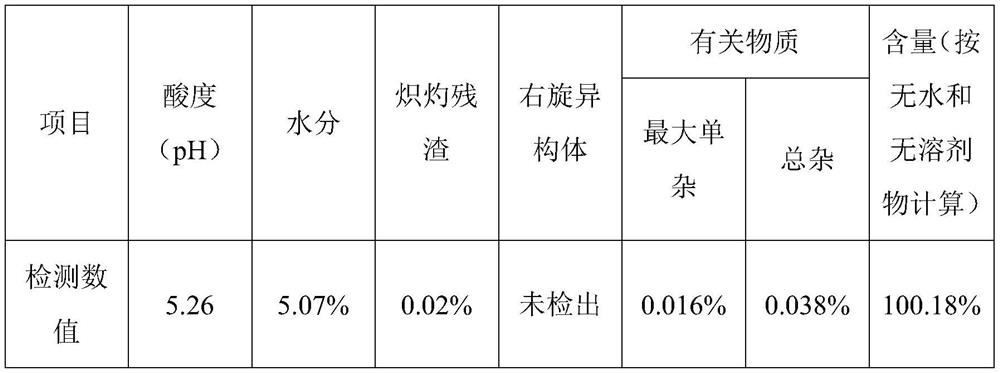
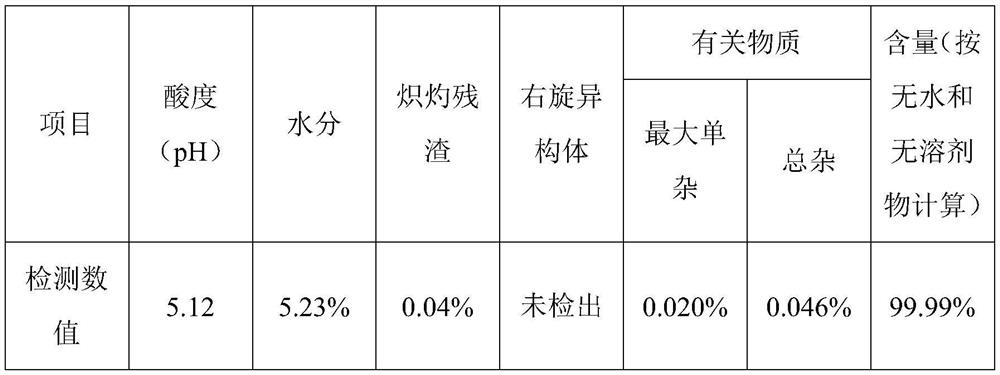
Industrial preparation method of ropivacaine hydrochloride monohydrate
A technology for ropivacaine hydrochloride and monohydrate, which is applied in the field of compound synthesis, can solve the problems of high risk of triphosgene use and storage, unsuitable for industrial production, and environmental pollution, and reduces the output and reaction of three wastes. The effect of short route and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] a. Prepare the DMF solution of 1-propylpiperidine-2-propyl carboxylate (3)
[0040] Add 500ml of DMF, 100g (0.77mol) of 2-piperidinecarboxylic acid, 237g (1.93mol) of 1-bromopropane and 319g (2.31mol) of potassium carbonate into a 2L four-necked flask, stir and raise the temperature to 70°C to 80°C, and control the The reaction was continued at low temperature for 2 to 3 hours. After TLC detected that the reaction was complete, the reaction was cooled to room temperature and suction filtered to obtain a DMF solution of 1-propylpiperidine-2-propyl carboxylate.
[0041] b. Preparation of N-(2,6-dimethylphenyl)-1-n-propylpiperidine-2-carboxamide (5)
[0042] Add 129.6g (1.16mol) potassium tert-butoxide and 400ml DMF to another 2L four-necked flask, stir until dissolved, add 112g (0.92mol) dimethylaniline at 20°C to 30°C with temperature control, and then raise the temperature to 90 ℃~100℃, add the DMF solution of 1-propylpiperidine-2-propyl carboxylate dropwise at this t...
Embodiment 2
[0051] a. Preparation of NMP solution of 1-propylpiperidine-2-propyl carboxylate (3)
[0052] Add 500ml NMP, 87g (0.67mol) 2-piperidinecarboxylic acid, 206.2g (1.68mol) 1-bromopropane, 277.5g (2.00mol) potassium carbonate and 6.6g (0.04mol) potassium iodide in 2L four-necked flask, stir Raise the temperature to 70°C-80°C, and control the temperature to continue the reaction for 1.5-3 hours. After TLC detected that the reaction was complete, the reaction was cooled to room temperature and suction filtered to obtain an NMP solution of propyl 1-propylpiperidine-2-carboxylate.
[0053] b. Preparation of N-(2,6-dimethylphenyl)-1-n-propylpiperidine-2-carboxamide (5)
[0054] Add 36.7g (0.94mol) sodium amide and 200ml NMP into another 2L four-neck flask, stir until dissolved, add 97.4g (0.80mol) dimethylaniline at 20°C to 30°C with temperature control, and then raise the temperature to 75°C At ~85°C, add the NMP solution of 1-propylpiperidine-2-propyl carboxylate dropwise at this t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com