A kind of preparation and purification method of ropivacaine hydrochloride intermediate
A technology for ropivacaine hydrochloride and intermediates, which is applied in the field of preparation of pharmaceutical compounds and can solve problems such as cumbersome processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of Example 1 Intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
[0048] Add L-piperidine formic acid hydrochloride (30.00g, 0.18mol) and toluene (300ml) successively into a clean 500ml three-necked reaction flask, stir, add N,N-dimethylformamide (1ml), chlorinated ethylene Sulfone (25.85g, 0.22mol). After the addition was completed, the temperature was raised to 50-55°C and the reaction was maintained for 3 hours. Add a buffer absorption device to vacuum for 1 hour. A toluene solution of 2,6-dimethylaniline (2,6-dimethylaniline (109.75 g, 0.91 mol) mixed with toluene (60 ml)) was added dropwise. After the addition, keep the reaction at 60°C for 2.0h. Cool down to 20-30°C, add purified water (300ml), separate layers, collect the water phase; add fresh toluene (300ml) to the water phase, adjust the pH of the system to 6-7 with 10% sodium hydroxide, separate layers, and collect the water phase Water phase is adjusted system pH=11~12 with ...
Embodiment 2
[0049] The refining of embodiment 2 intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
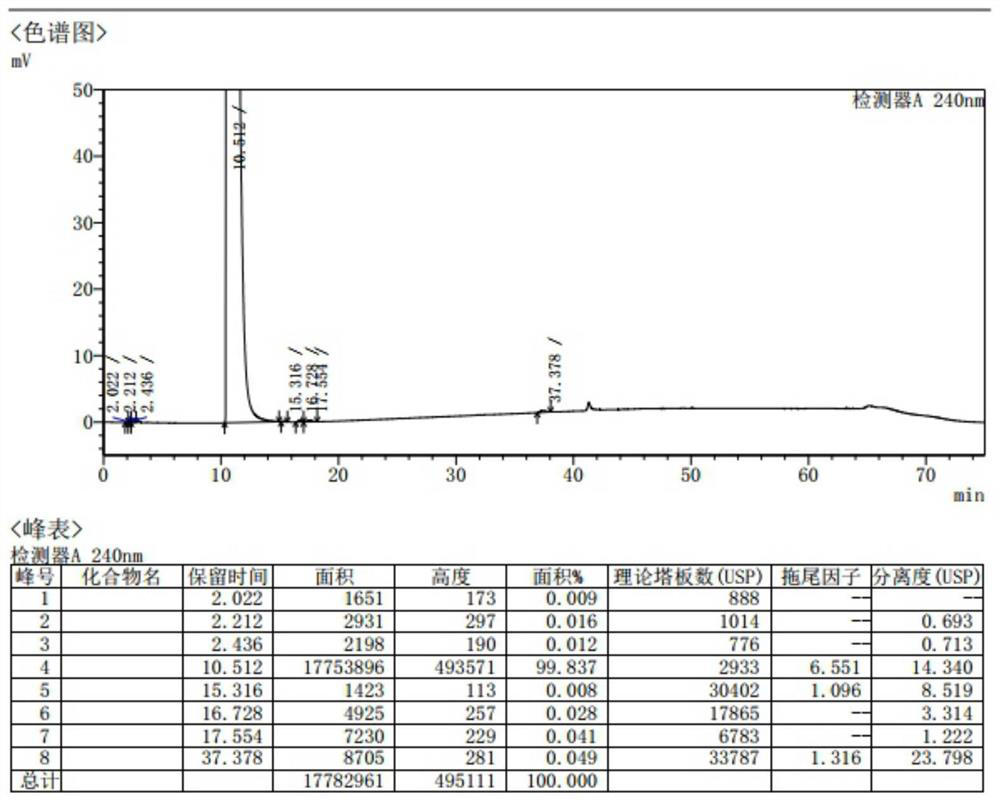
[0050] The intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide (5.00g, 21.52mmol) obtained in Example 1 was sequentially added to a 100ml clean three-necked reaction flask , diethyl ether (50ml), stir and heat up to reflux, reflux for 1 hour and keep stirring for 1 hour, cool down to room temperature, keep stirring for 1 hour, filter with suction, diethyl ether (10ml) rinse the filter cake, and dry the filter cake in a 50°C blast oven for 2 hours 2.66g (yield 53.2%, calculate HPLC purity 99.837% according to peak area normalization method), spectrum sees attached figure 1 .
Embodiment 3
[0051] The purification of embodiment 3 intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
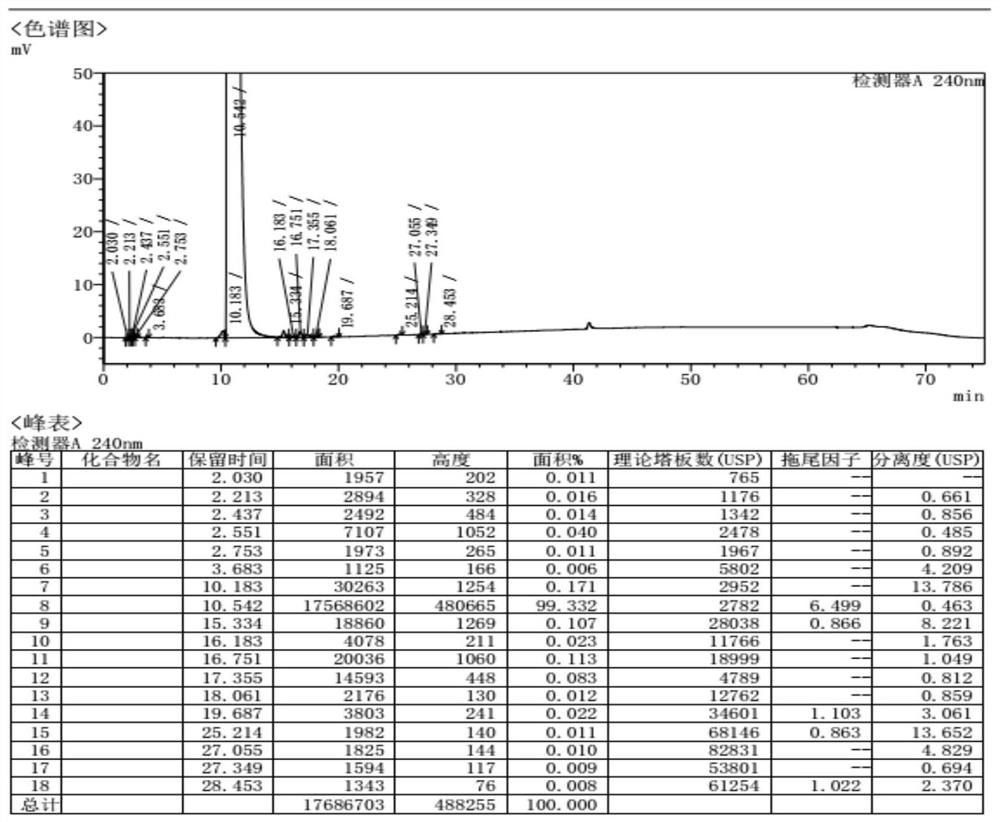
[0052]The intermediate (-)-(2S)-N-(2,6-dimethylphenyl)piperidine-2-carboxamide (5.00g, 21.52mmol) obtained in Example 1 was sequentially added to a 100ml clean three-necked reaction flask , isopropyl ether (50ml), stir and heat up to reflux, reflux and heat preservation and stirring for 1 hour, cool to room temperature, heat preservation and stirring for 1 hour, suction filtration, isopropyl ether (10ml) rinse the filter cake, and blow the filter cake at 50 ° C Dry in a drying oven for 2 hours to 3.45 g (yield 69.0%, HPLC purity 99.332% calculated by peak area normalization method). See attached figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com