A kind of ropivacaine hydrochloride impurity and preparation method thereof
A technology for ropivacaine hydrochloride and impurities, which is applied in the field of ropivacaine hydrochloride impurities and its preparation, can solve the problems affecting drug efficacy, toxic and side effects, and affecting the purity of ropivacaine hydrochloride, and reduce the quality control analysis The effect of cost, simple operation, and excellent anesthesia performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
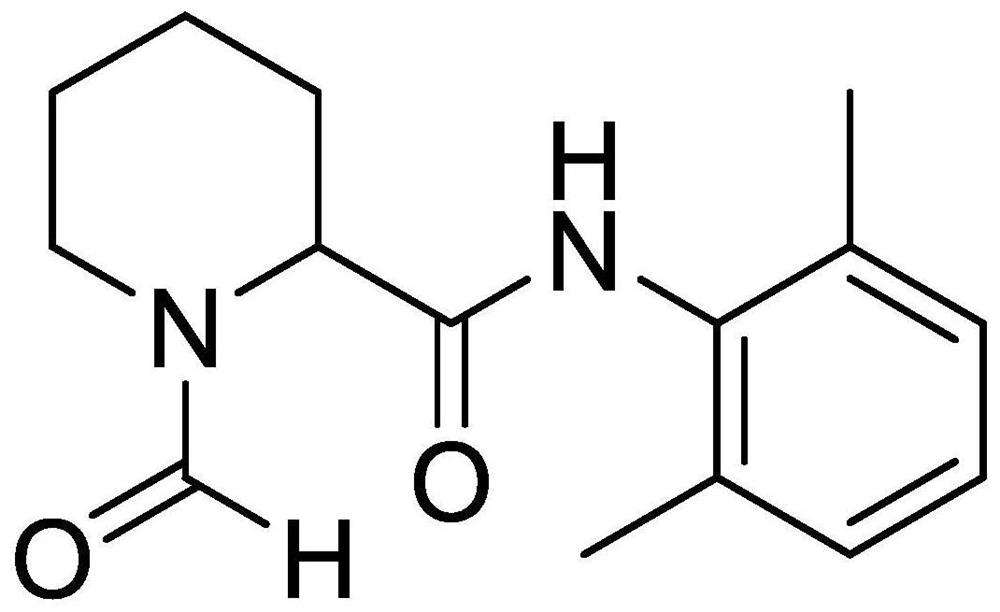
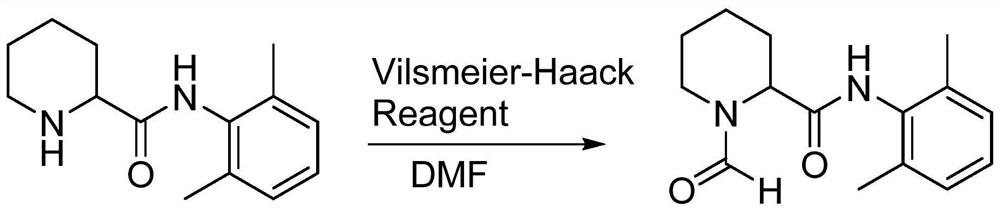
[0030] The present invention also provides a method for preparing impurity ropivacaine hydrochloride with the structure shown in formula I, said method comprising the steps of: performing Vilsmeier-Haack reaction on the compound shown in formula II;
[0031]
Embodiment 1
[0033] A kind of preparation method of ropivacaine hydrochloride impurity as the embodiment of the present invention, described method comprises steps:
[0034] (1) Add 20ml dimethylformamide (DMF) and 10.2g (0.085mol) thionyl chloride in the reaction flask, add N-(2,6-dimethylphenyl) piperidine-2-carboxamide ( 10.0g, 0.043mol) of DMF solution 20ml, stirred until the end of the reaction, and determined the end point of the reaction by spot plate method;
[0035] (2) add water to the reaction system after step (1) to quench, adjust pH=14 with sodium hydroxide solution, add chloroform to extract;
[0036] (3) The organic phases were combined, spin-dried, concentrated to dryness after passing through the column to obtain 4.6 g of off-white solid powder with a purity of 98.9%.
[0037] Carry out NMR identification to the product, the result is: 1 H-NMR (CDCl 3 , 400MHz): δ1.4-1.8(6H,m), 2.20(6H,s), 3.4-3.6(2H,m), 5.2(1H,d), 7.0(3H,m), 7.4(1H,s ), 8.2 (1H, s); the product is de...
Embodiment 2
[0041] A kind of preparation method of ropivacaine hydrochloride impurity as the embodiment of the present invention, described method comprises steps:
[0042] (1) Add 20ml dimethylformamide (DMF) and 10.2g (0.085mol) thionyl chloride in the reaction flask, add N-(2,6-dimethylphenyl) piperidine-2-carboxamide ( 10.0g, 0.043mol), stirred until the end of the reaction;
[0043] (2) add water to the reaction system after step (1) to quench, adjust pH=13 with sodium hydroxide solution, add ethyl acetate for extraction;
[0044] (3) The organic phases were combined, spin-dried, dissolved by adding 25ml of methyl isobutyl ketone, cooled to 10-20°C and crystallized to obtain 4.8g of off-white solid powder.
[0045] Carry out NMR identification to the product, the result is: 1 H-NMR (CDCl 3, 400MHz): δ1.4-1.8(6H,m), 2.20(6H,s), 3.4-3.6(2H,m), 5.2(1H,d), 7.0(3H,m), 7.4(1H,s ), 8.2 (1H, s); the product is detected by liquid phase mass spectrometry, and the result is m / z: 261.2 [M+H]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com