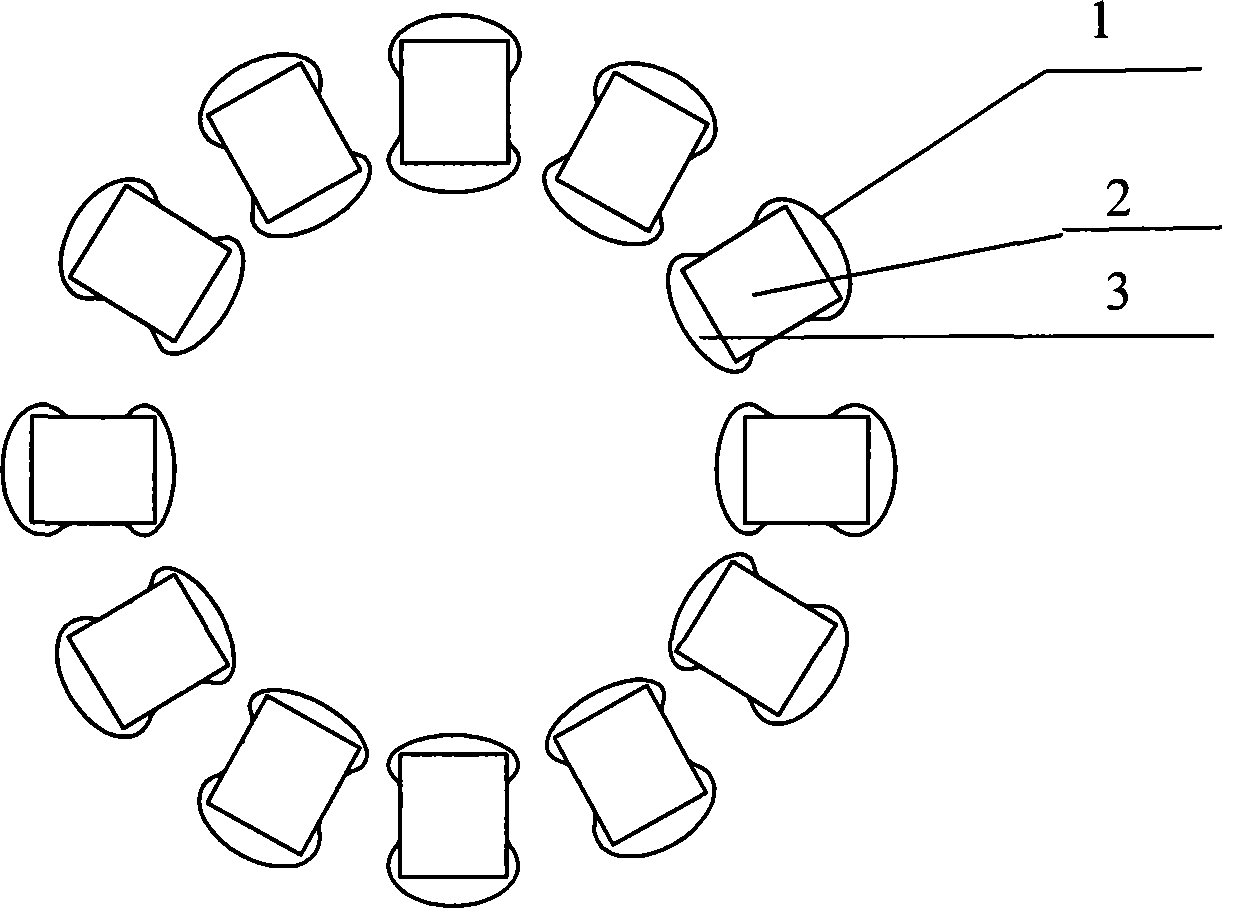
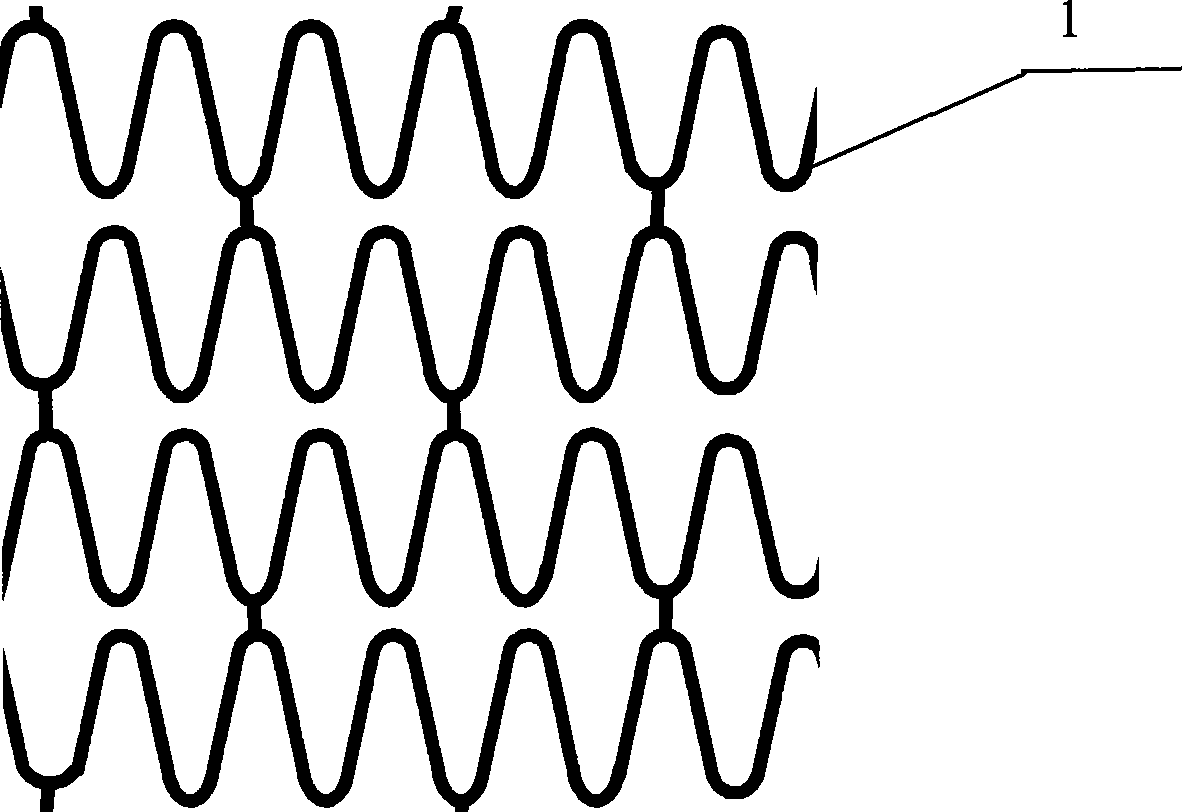
Blood vessel support for curing coronary heart disease
A vascular stent and coronary heart disease technology, applied in the field of vascular stents for the treatment of coronary heart disease, can solve problems such as distribution limitations, and achieve the effects of avoiding thrombosis, optimizing functions, and inhibiting the proliferation of smooth muscle cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the preparation of vascular stent of the present invention
[0035] Taking the 2.5mm×14mm bracket as an example to introduce the manufacturing process and main parameters of the bracket:
[0036] Add 10.0g of polylactic acid (racemic polylactic acid) with a viscosity of 0.9dl / g to 4.5g of rapamycin, put it in a clean beaker, add 100ml of ethyl acetate at 0-60°C at 500 rpm Minute mixing for 10 minutes, and then ultrasonic at 20KHZ for 20 minutes, the resulting solution is the coating solution, that is, the coating solution on the side of the bare metal stent in contact with the blood vessel wall.
[0037] Add 10.0 g of polylactic acid (racemic polylactic acid) with a viscosity of 0.9 dl / g to 1.0 g of pcDNA3-VEGF165 plasmid (purchased from Beijing Yili Hi-Tech Biotechnology Research Institute Co., Ltd., pcDNA3-VEGF 165 is based on pcDNA3.1 Departure plasmid, the recombinant plasmid capable of expressing human VEGF165 obtained by inserting the coding gene fr...
Embodiment 2
[0043] Embodiment 2, preparation of vascular stent of the present invention
[0044] Taking the 2.75mm×14mm bracket as an example to introduce the manufacturing process and main parameters of the bracket:
[0045] Add 2.5g rapamycin to 10.0g polylactic acid (racemic polylactic acid) with a viscosity of 0.2dl / g, put it in a clean beaker, add 100ml of acetone at 0-60°C at 500 rpm Mixing for 10 minutes, and then ultrasonicating at 20KHZ for 20 minutes, the resulting solution is the coating solution, that is, the coating solution on the side of the bare metal stent contacting the blood vessel wall is prepared.
[0046]Add 0.03g of pcDNA3-VEGF165 plasmid (purchased from Beijing Yili Hi-Tech Biotechnology Research Institute Co., Ltd., to 10.0g of polylactic acid with a viscosity of 0.2dl / g, pcDNA3-VEGF165 is a plasmid starting from pcDNA3.1 and inserted into human VEGF165 A recombinant plasmid that can express human VEGF165 obtained from the coding gene fragment), add 100ml of acet...
Embodiment 3
[0052] Embodiment 3, the preparation of vascular stent of the present invention
[0053] Taking the 3.0mm×14mm bracket as an example to introduce the manufacturing process and main parameters of the bracket:
[0054] Add 10.0g of polylactic acid with a viscosity of 1.2dl / g to 15g of rapamycin, put it in a clean beaker, add 100ml of chloroform, mix at 500 rpm for 10 minutes at 0-60°C, and then sonicate at 20KHZ After 20 minutes, the obtained solution is the coating solution, that is, the coating solution on the side where the bare metal stent contacts the blood vessel wall.
[0055] Add 10.0 g polylactic acid with a viscosity of 1.0 to 10.0 g pcDNA3-VEGF165 plasmid (purchased from Beijing Yili Hi-Tech Biotechnology Research Institute Co., Ltd., pcDNA3-VEGF165 is obtained by inserting the coding gene fragment of human VEGF165 from pcDNA3.1 A recombinant plasmid that can express human VEGF165), add 100ml of chloroform and mix at 500 rpm at 0-60°C for 10 minutes, then ultrasonica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com