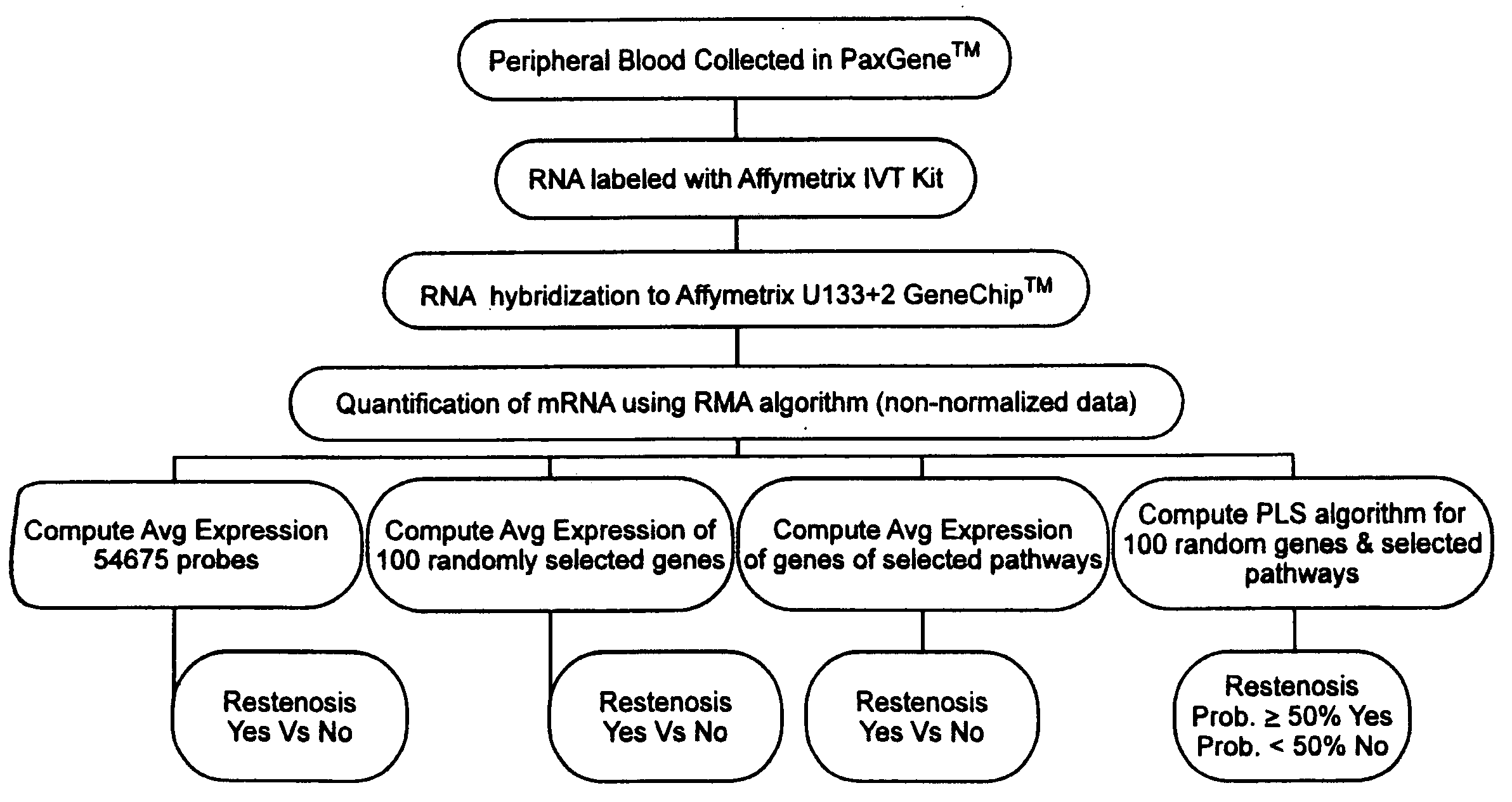
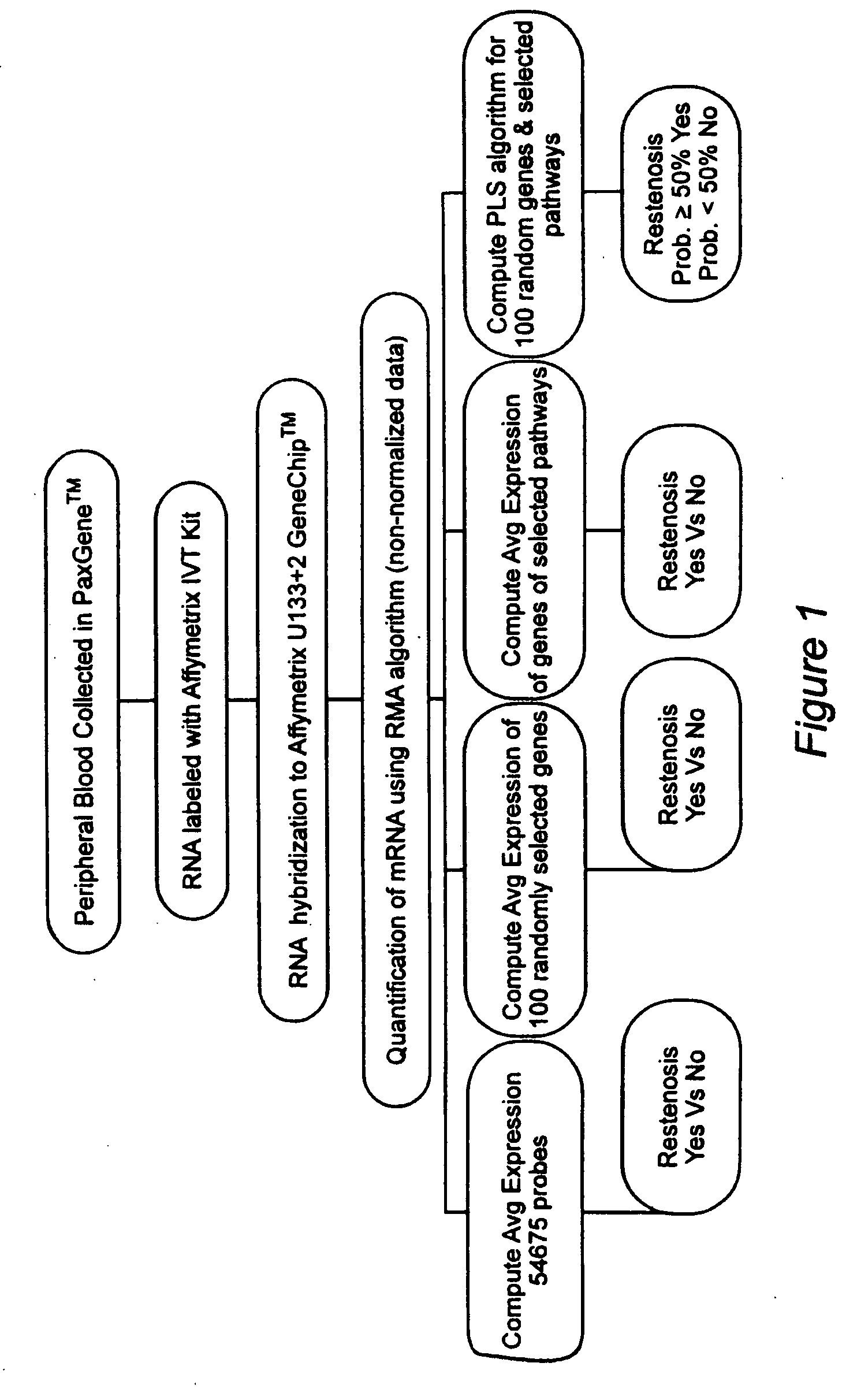
Prediction of bare metal stent restenosis
probability prediction technology, applied in the field of predicting the probability of restenosis, can solve the problems of insufficient prediction of coronary stenting success, inability to accurately predict the risk factors of restenosis, and high cost of using a drug-coated stent instead of a bare metal stent, so as to reduce the risk of major adverse clinical events, reduce costs, and increase gene activation/up-regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Assessment of mRNA Expression in Patients Receiving Bare Metal Stents and Incidence of Restenosis
[0031]Summary: One hundred twelve (112) non-diabetic patients were enrolled in a prospective clinical trial designed to test the hypothesis that an mRNA expression profile can accurately predict which patients will restenose after bare metal stent placement. Peripheral blood samples were collected prior to coronary angioplasty in all patients. Fifty-six (56) patients were found to have significant coronary artery disease and received at least one bare metal stent. All patients had follow-up angiograms 6 months post stent placement. Twenty-three patients experienced restenosis within 6 months of stent placement. Whole blood mRNA expression profiling was performed using the Affymeytrix U133+2 GeneChip™. Putative molecular pathways and functional molecular families responsible for bare metal stent restenosis were pre-specified prior to analysis. A partial least squares algorithm was used to...
example 2
Methods for Predicting a Patient's Propensity for Restenosis
[0053]Based on the data in Tables 12A, 12B and 12C above, the patient may be predicted to be a good candidate for a bare metal stent (one not likely to have restenosis within six months) according to the following three exemplary methods:
[0054]a) In a first embodiment, the invention provides a method for predicting a patient's propensity for bare metal stent restenosis, comprising the steps of: ai) obtaining a whole blood sample from a patient deemed to be in need of a stent; aii) measuring total mRNA from a volume of blood obtained from said blood sample using a gene probe substrate having at least a plurality of gene probes that hybridize to at least a plurality of mRNA sequences; aiii) computing an average mRNA value for said predetermined volume of blood from said total mRNA and said plurality of genes; aiv) classifying said patient as having a prognosis selected from a group consisting of a first prognosis and a second...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cell adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com