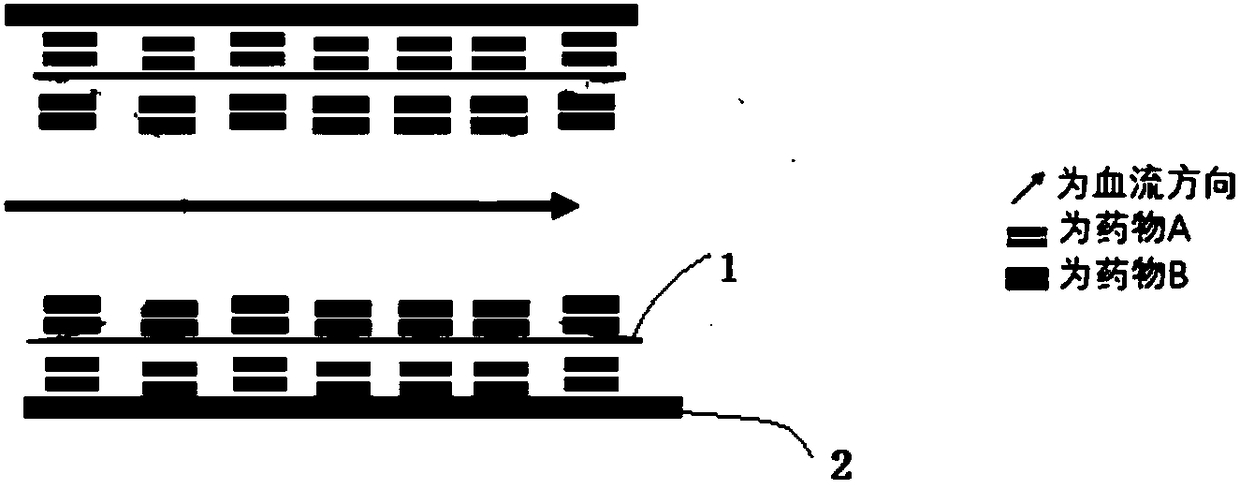
Bidirectional double-drug eluting stent and preparation method thereof
An eluting stent and dual-drug technology, applied in the field of eluting stents, can solve problems such as lumen loss, stent thrombosis, and stent stenosis, and achieve the effects of accelerating re-endothelialization, rapid endothelialization, and delayed endothelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The selected base stent is a cobalt alloy stent, and a solution with the following components is configured: the carrier material is polylactic acid, the solvent of atorvastatin is a mixed solvent of acetone and ether (mass ratio 2:8), and the solvent of tacrolimus is chloroform Mixed solvent with ethanol (mass ratio 1:1).
[0024] Polylactic acid (Mn=50000) 0.1g
[0025] Atorvastatin 1mg
[0026] Tacrolimus 20mg
[0027] Acetone 20ml + ether 80ml
[0028] Chloroform 50ml+ and ethanol 50ml
[0029] Mix the above systems separately and stir at 10 rpm to form a solution.
[0030] Clean the cobalt alloy stent and place it on an ultrasonic atomization sprayer. First, spray the atorvastatin / polylactic acid solution on the inside of the stent. The ultrasonic power is 0.1W, the solution flow rate is 0.01ml / min, and the number of sprays is 10 times. Finish drying at room temperature for 0.1 hour, and then spray tacrolimus / polylactic acid solution on the outside of the sten...
Embodiment 2
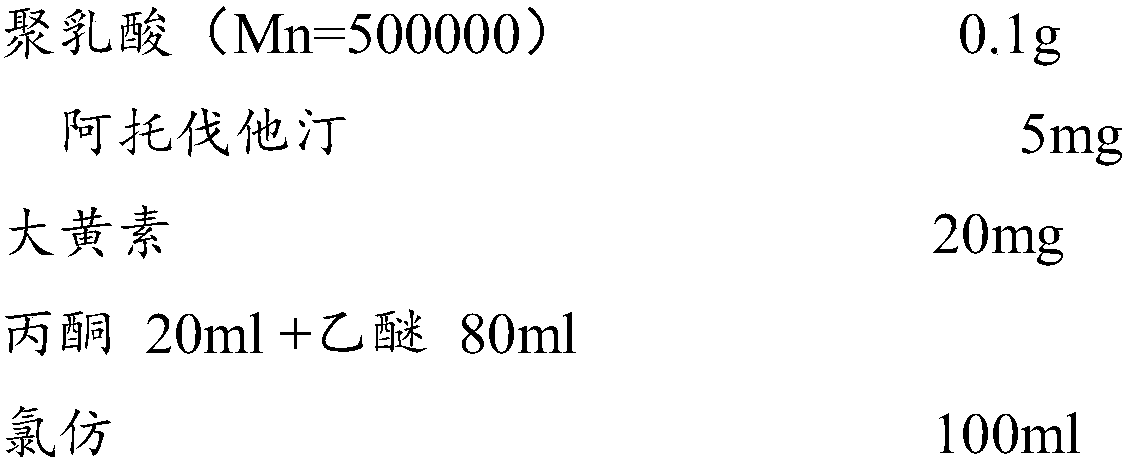
[0032] The selected substrate stent is a cobalt alloy stent, and a solution with the following components is configured: the carrier material is polylactic acid, the solvent of atorvastatin is a mixed solvent of acetone and ether (mass ratio 2:8), and the solvent of emodin is chloroform.
[0033]
[0034] Mix the above systems separately and stir at 300rpm to form a solution.
[0035] Clean the cobalt alloy stent and place it on an ultrasonic atomization sprayer. First, spray the atorvastatin / polylactic acid solution on the inside of the stent. The ultrasonic power is 20W, the solution flow rate is 0.5ml / min, and the number of sprays is 20 times. The spraying is completed. Dry at room temperature for 4 hours, and then use an ultrasonic atomization sprayer to spray the emodin / polylactic acid solution on the outside of the bracket. The ultrasonic power is 20W, the solution flow rate is 0.1ml / min, and the number of sprays is 30 times. After spraying, put the bracket on the Vac...
Embodiment 3
[0037] The selected base stent is a magnesium alloy stent, and a solution with the following components is configured: the carrier material of fluvastatin is polylactic acid-glycolic acid copolymer, and the solvent of fluvastatin is a mixed solvent of tetrahydrofuran and ether (mass ratio 9:1); The carrier material of mycin is polyglycolic acid, and the solvent is acetone.
[0038]
[0039] Mix the above systems separately and stir at 600rpm to form a solution.
[0040] Clean the magnesium stent and place it on an ultrasonic atomization sprayer. First, spray the fluvastatin / polylactic acid-glycolic acid copolymer solution on the inside of the stent. The ultrasonic power is 10W, the solution flow rate is 0.1ml / min, and the number of sprays is 40 times. After spraying, dry at room temperature for 4 hours, and then use an ultrasonic atomization sprayer to spray the rapamycin / polyglycolic acid solution on the outside of the bracket. The ultrasonic power is 10W, the solution flo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com