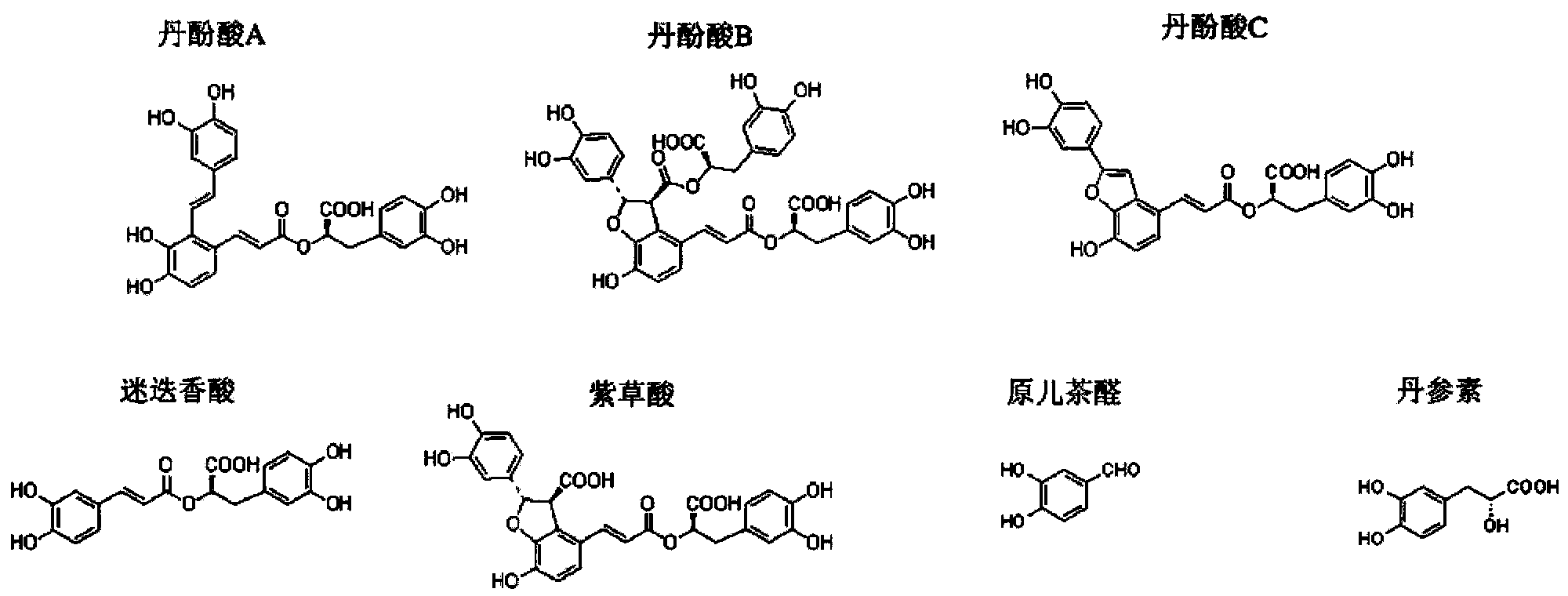
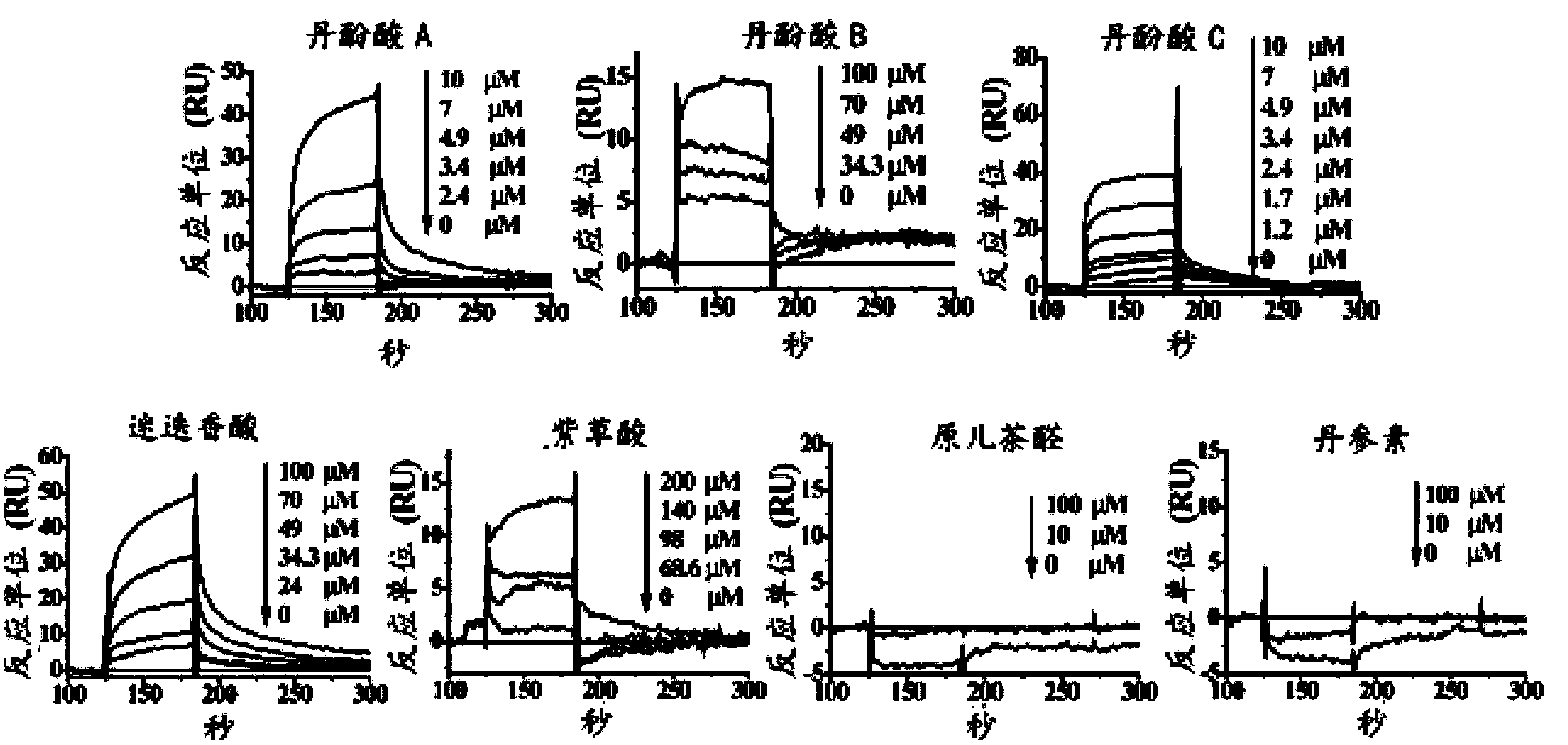
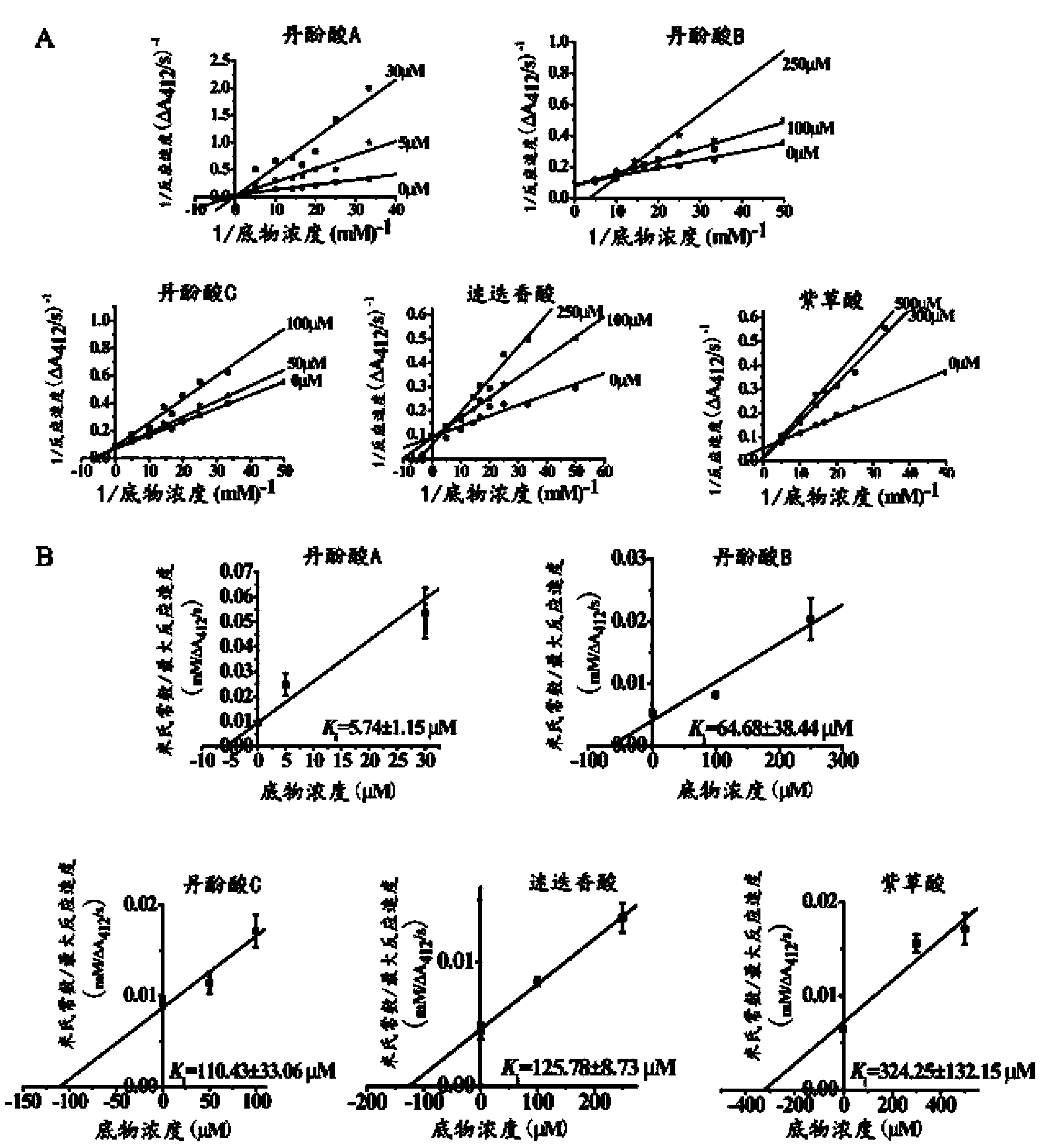
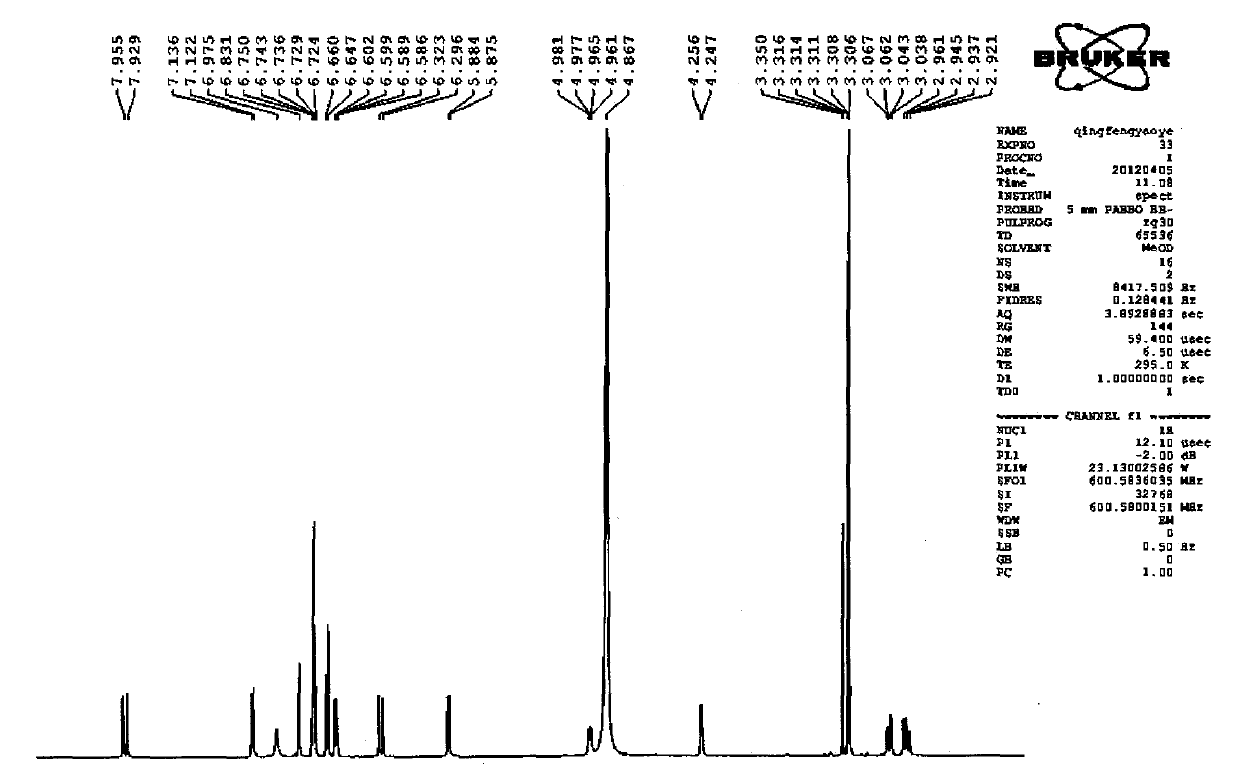
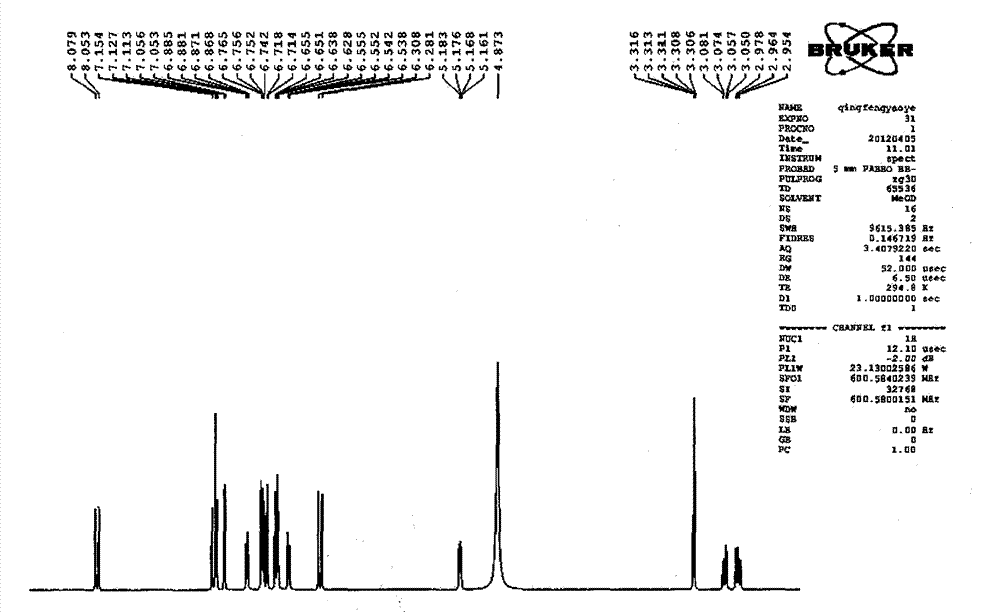
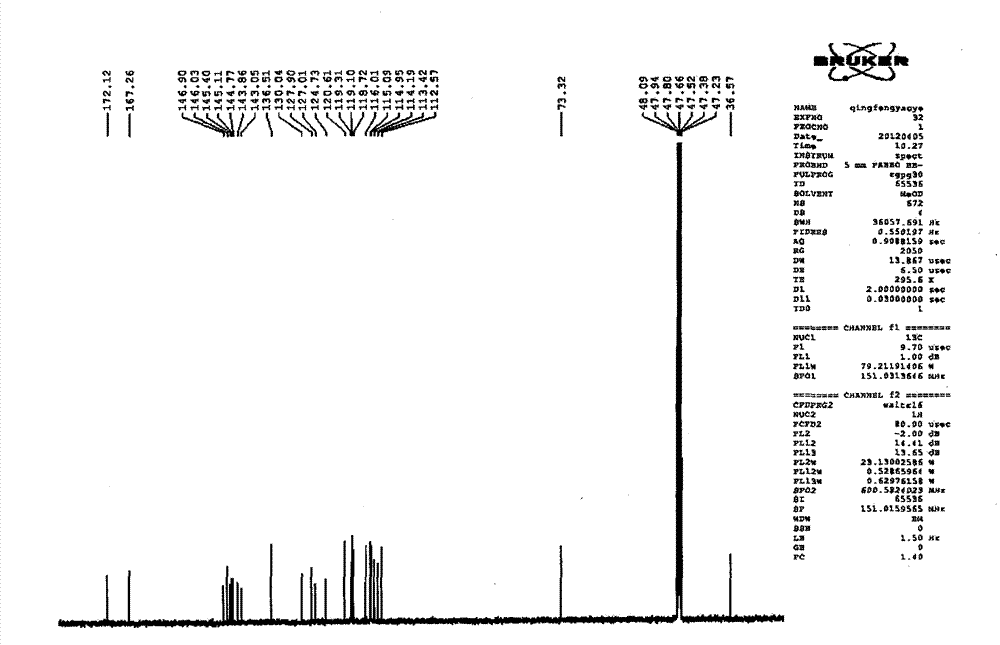
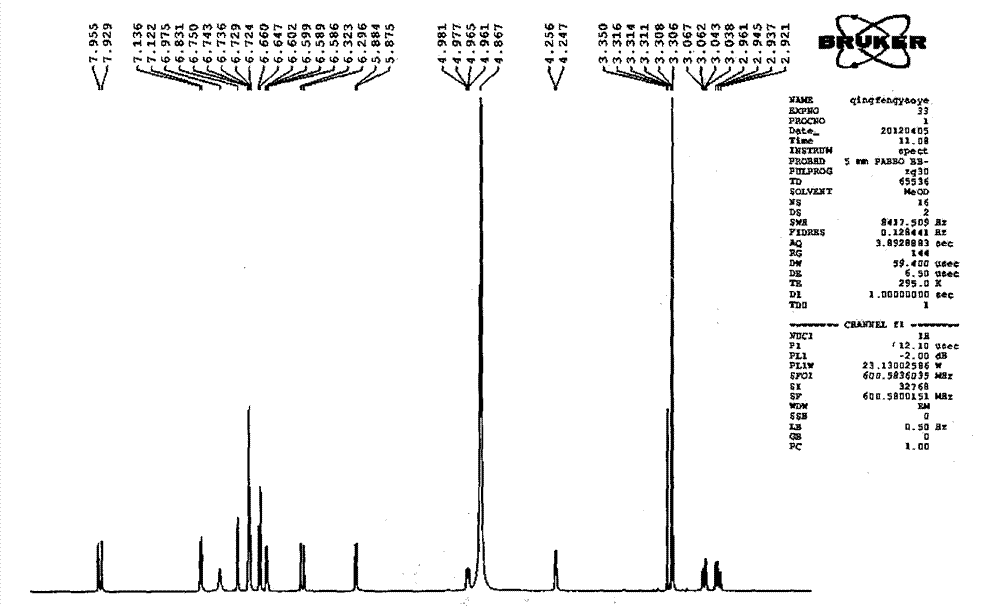
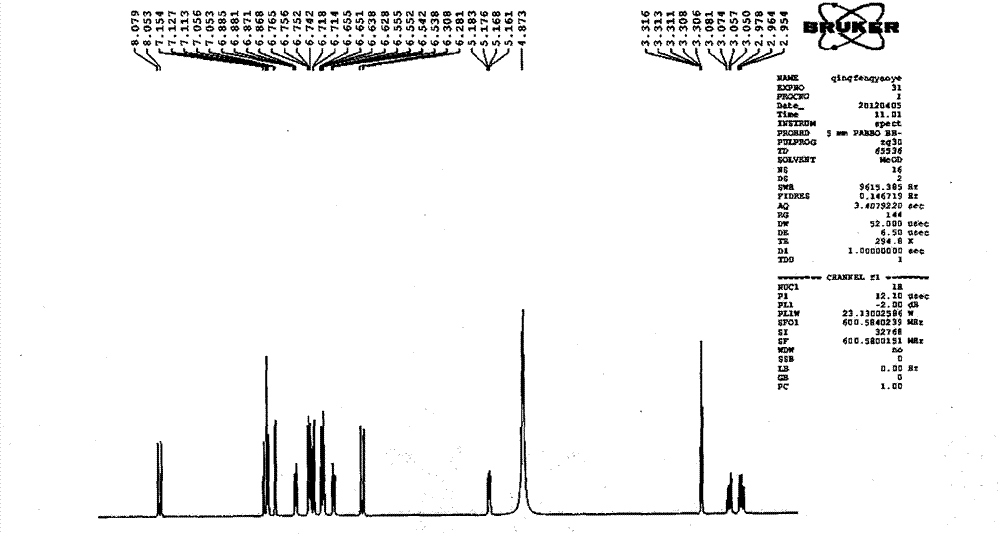
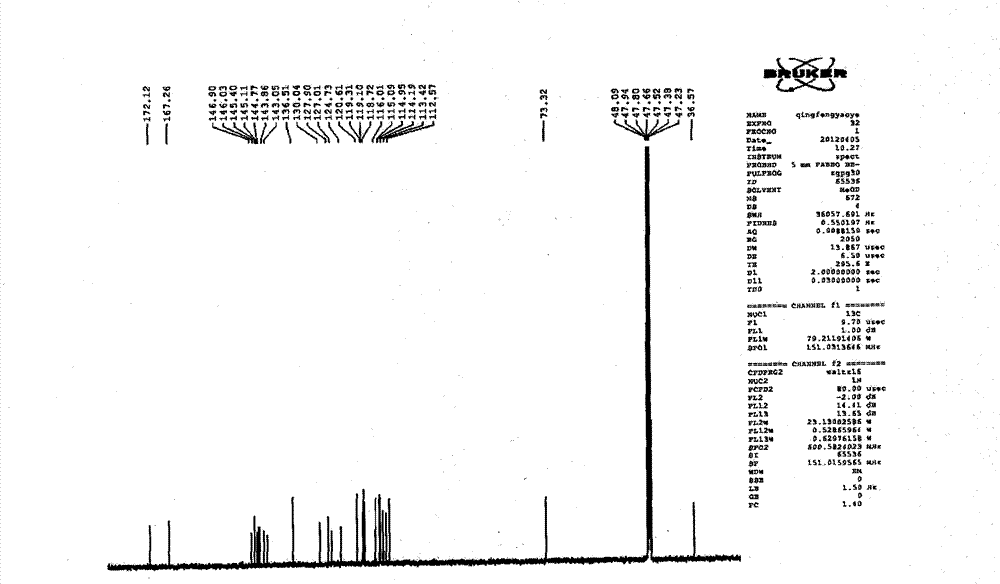
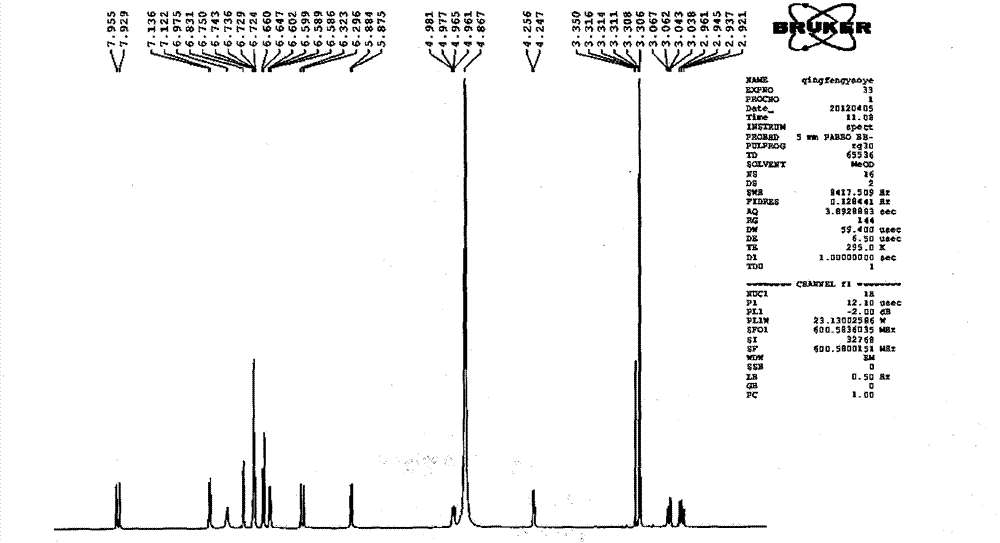
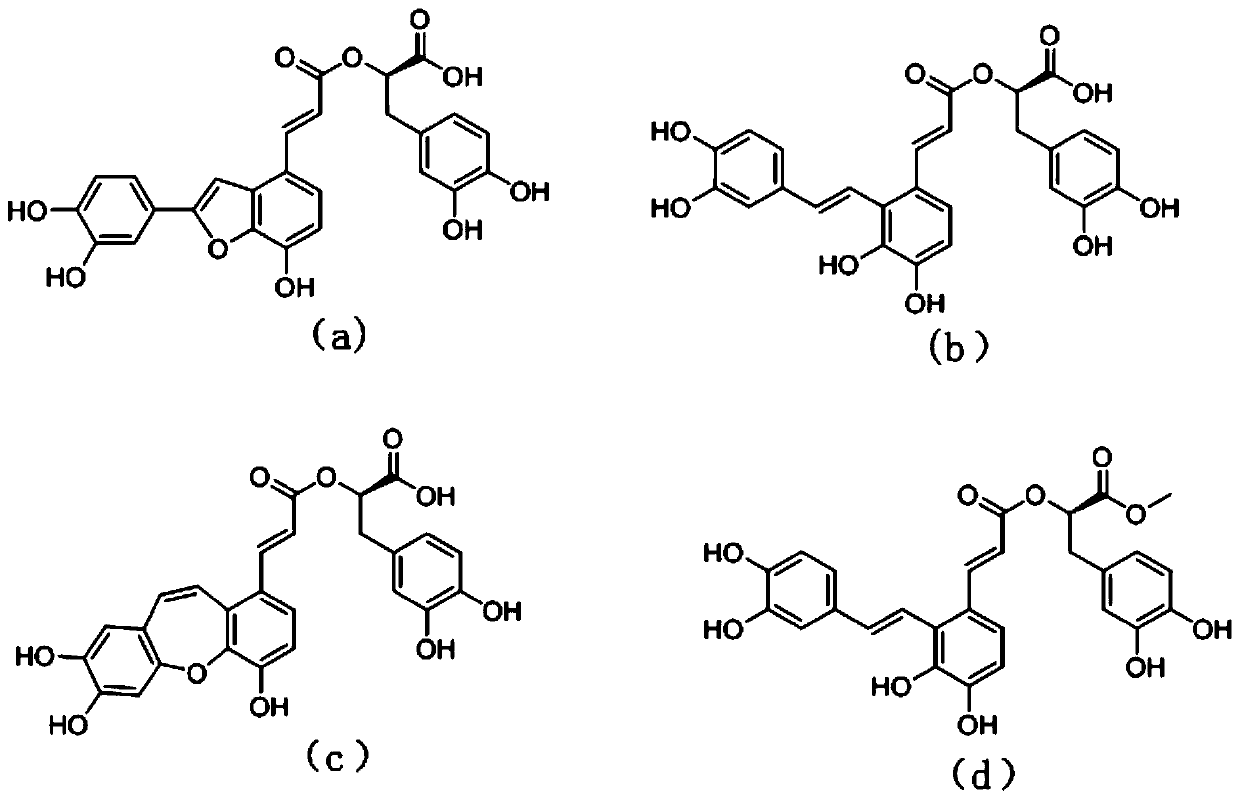
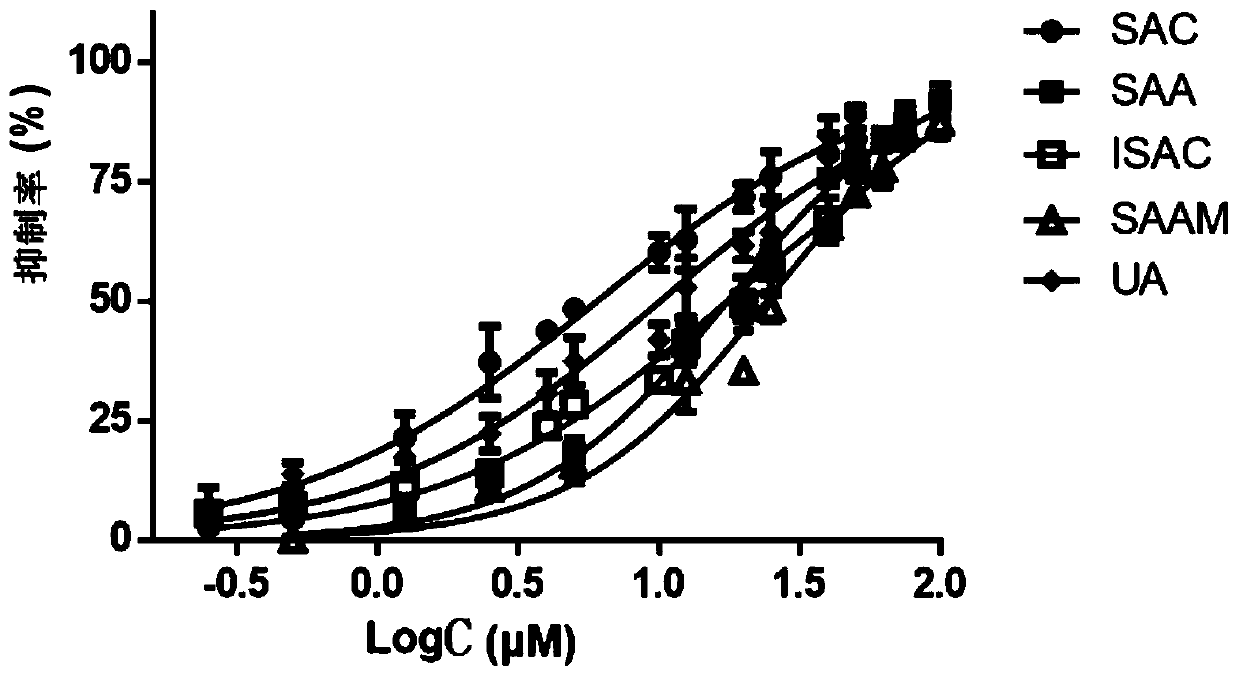
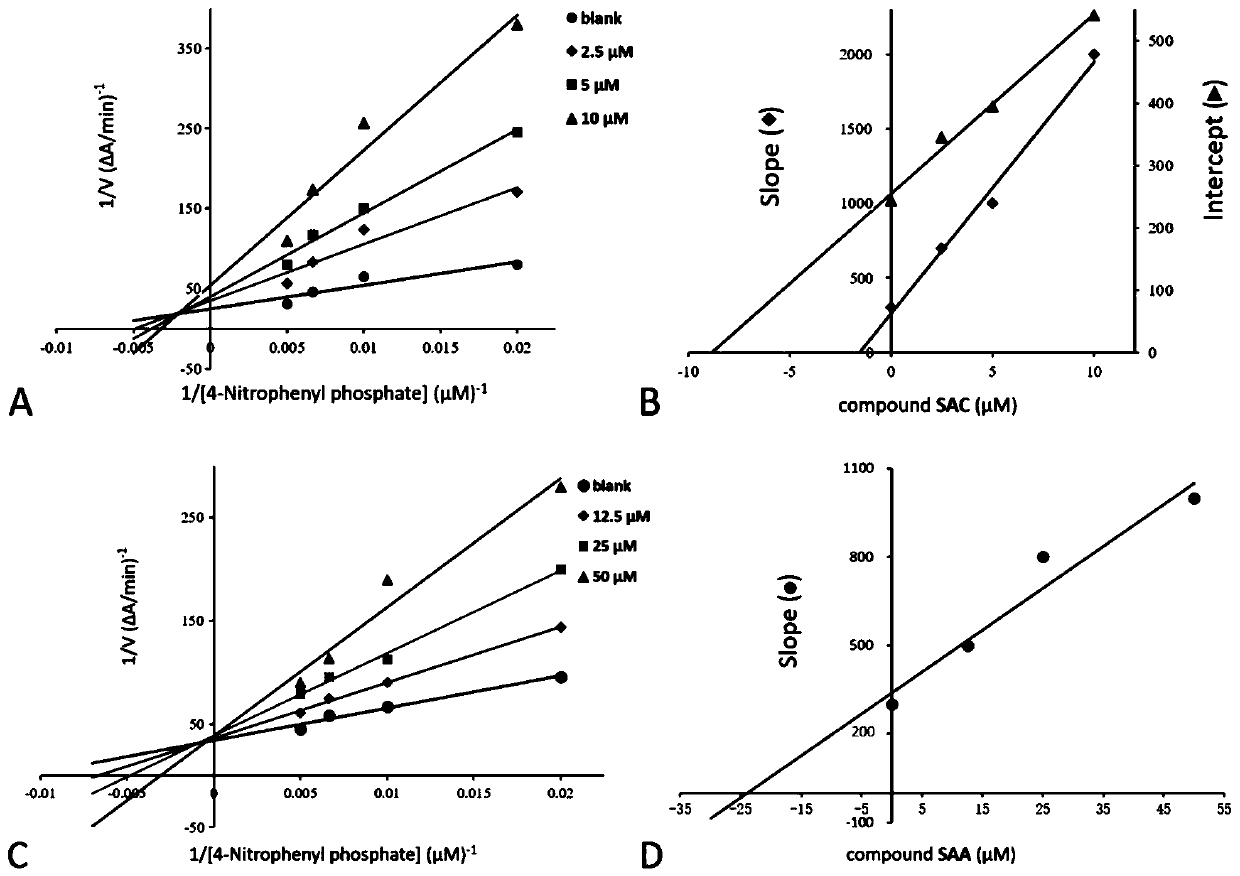
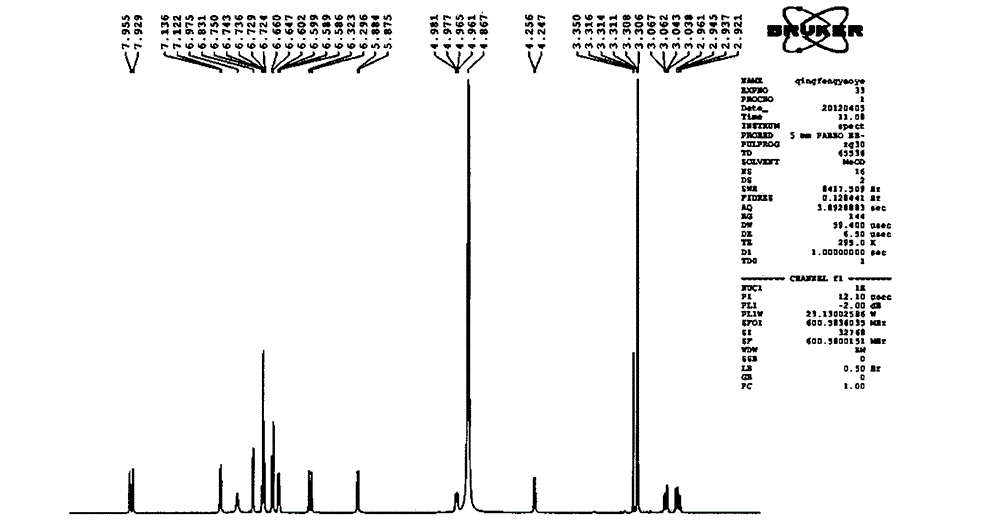
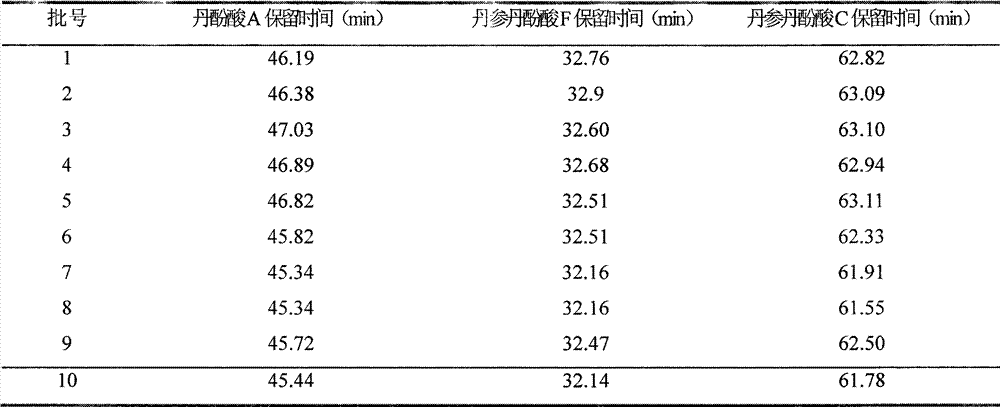
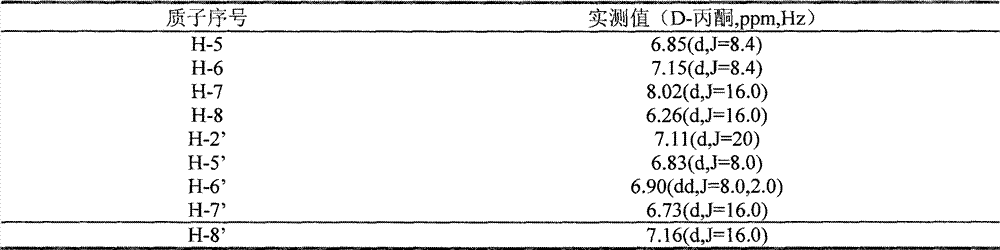
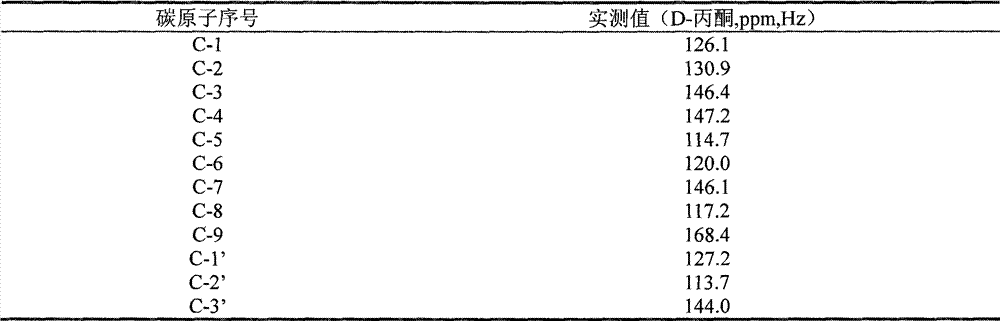
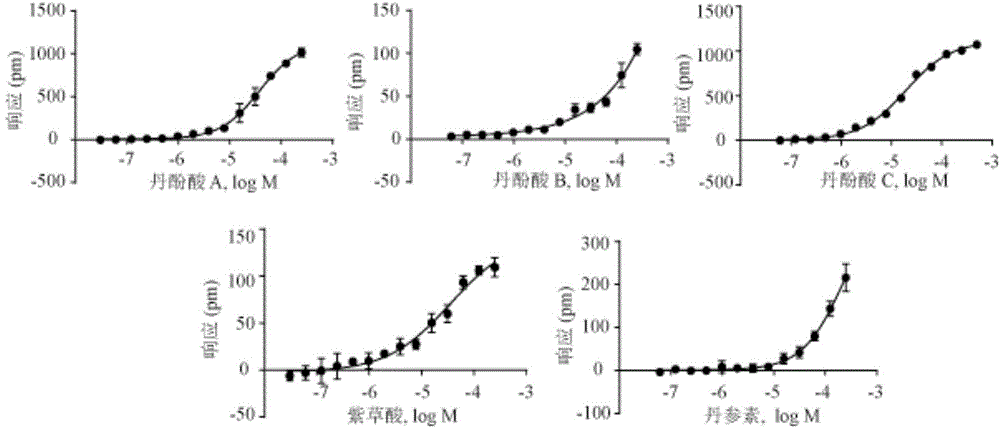
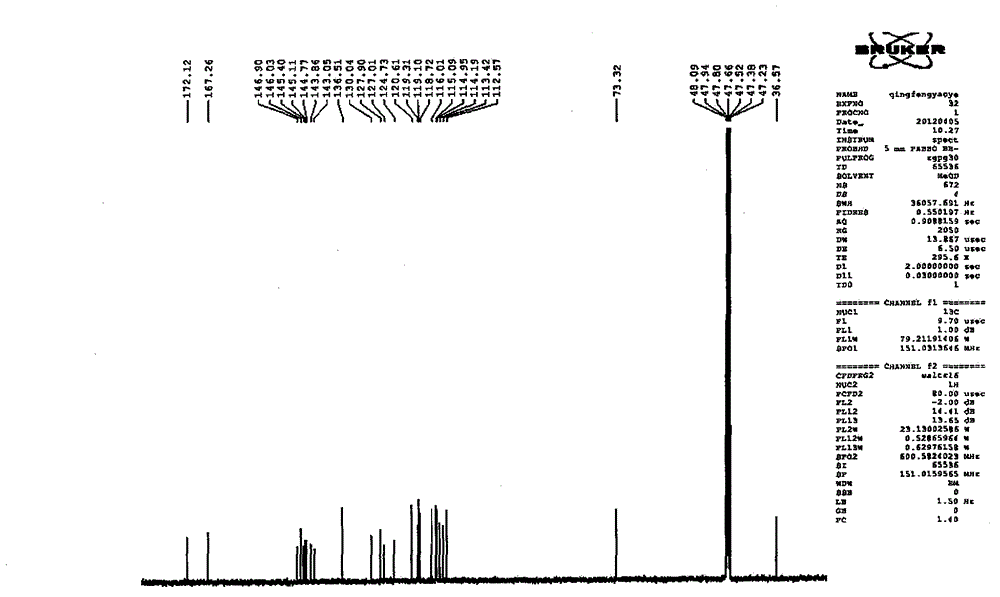
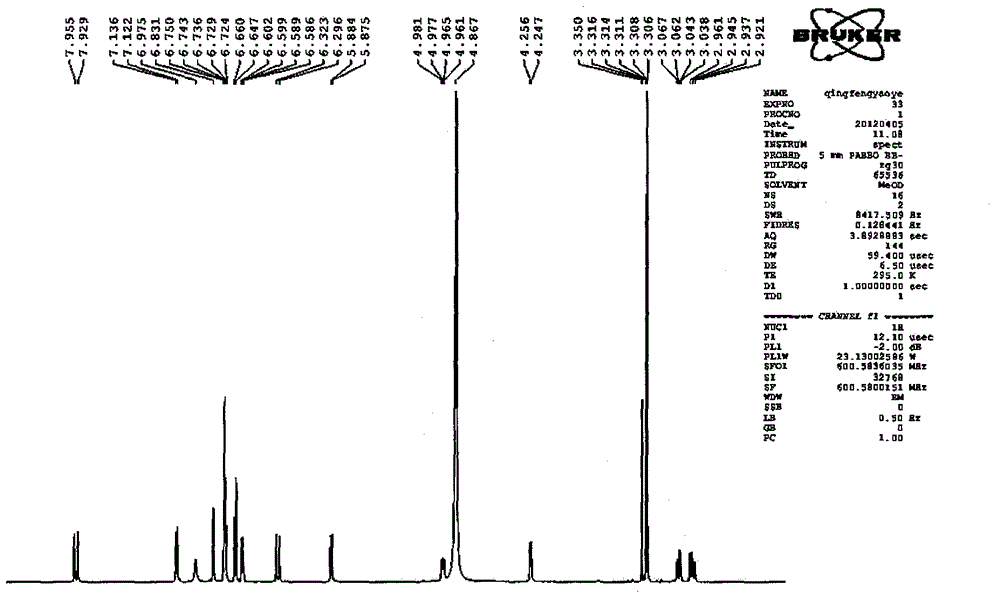
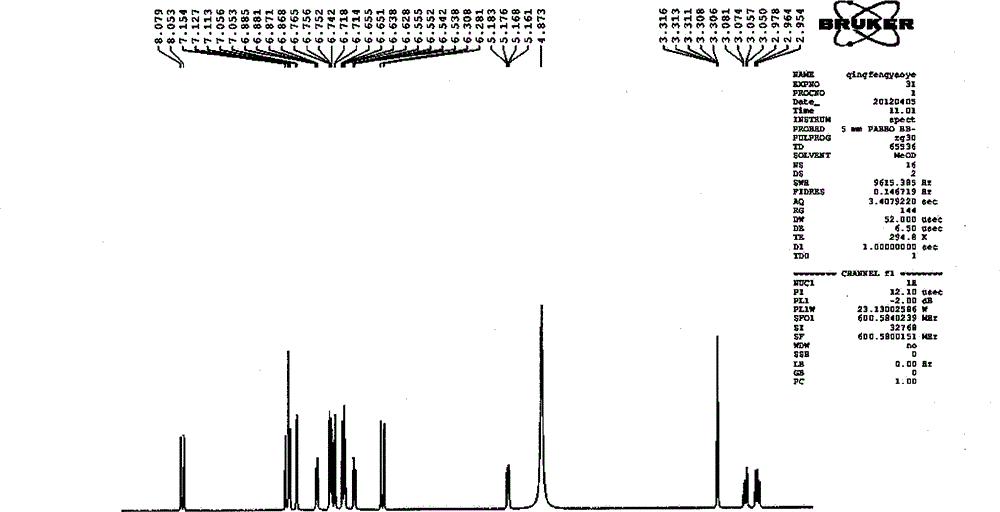
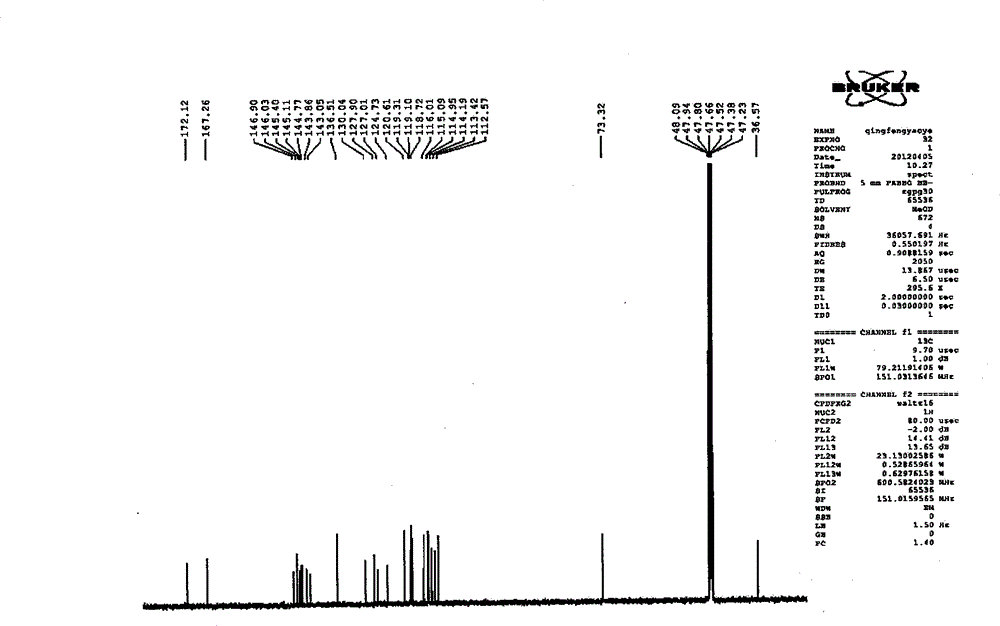
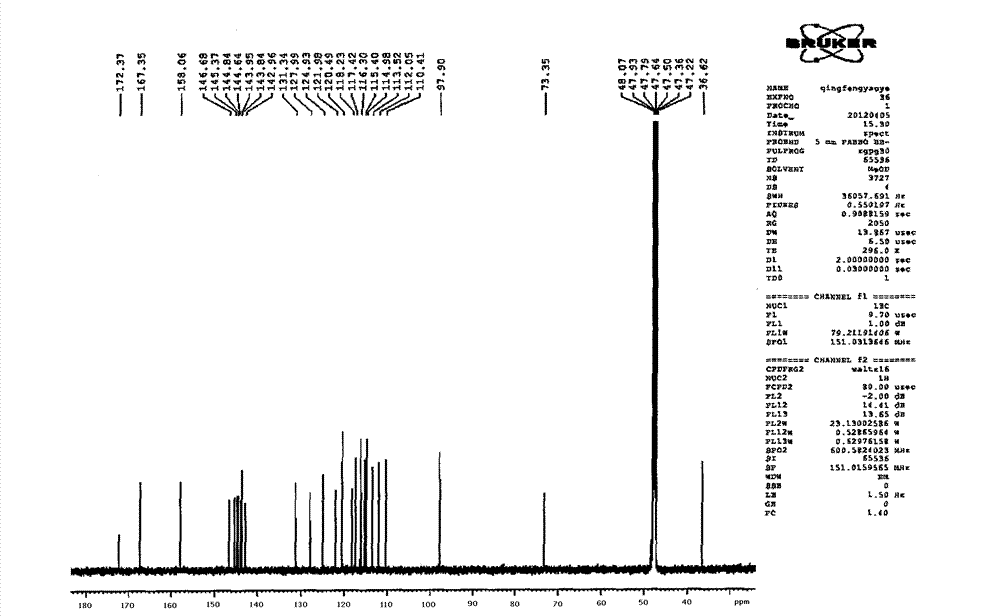
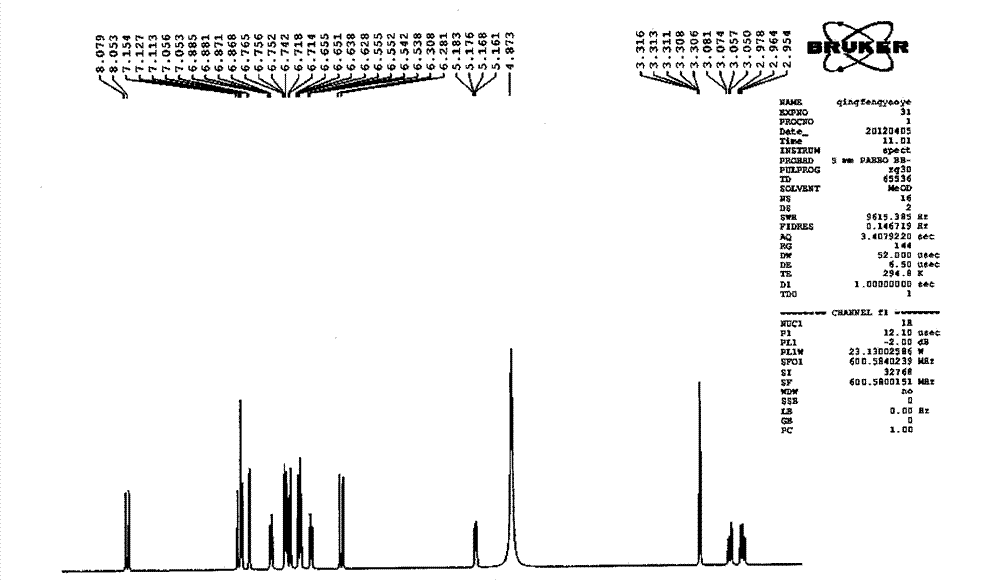
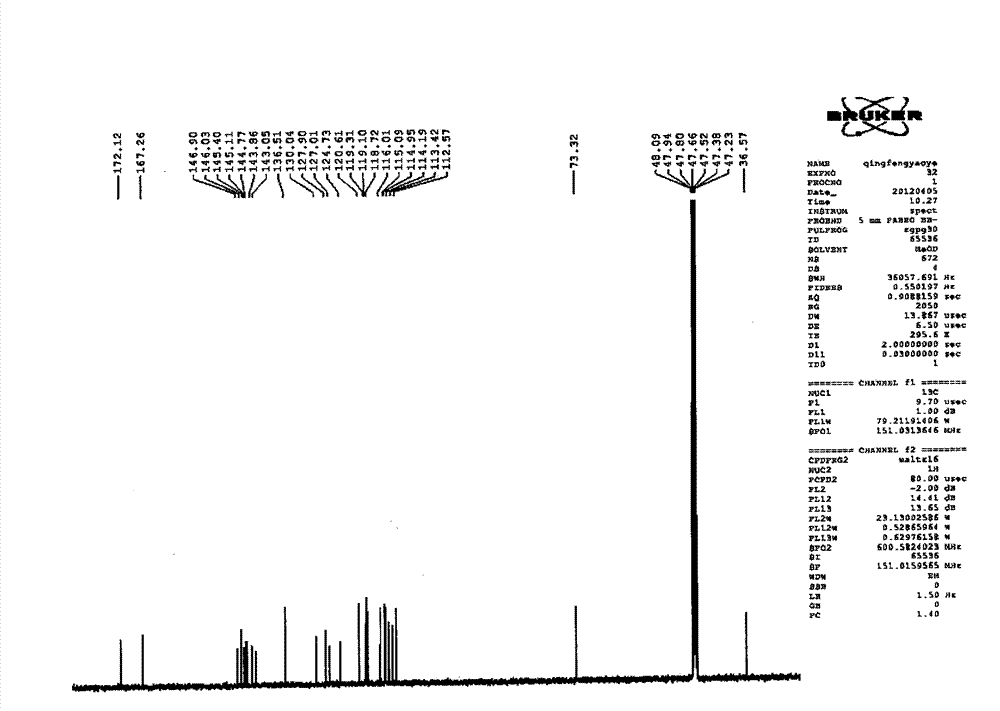
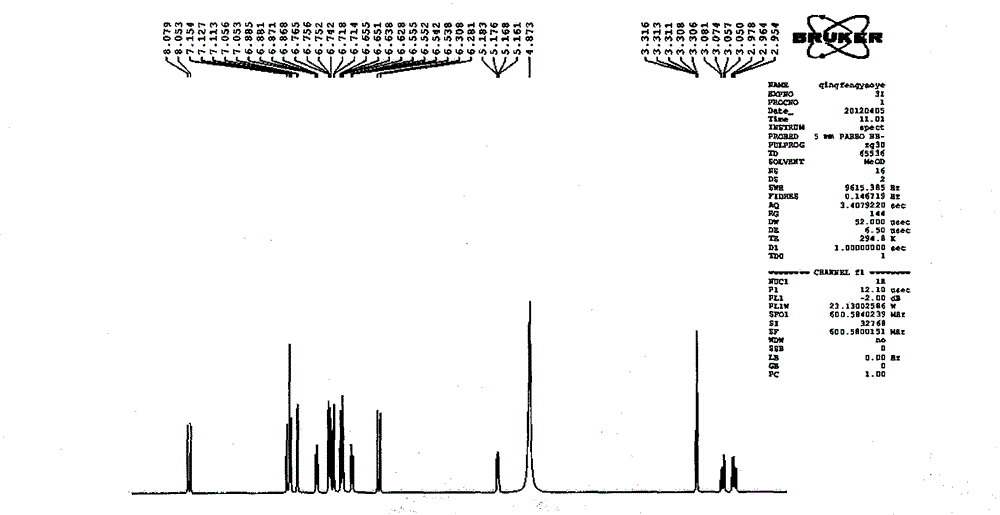
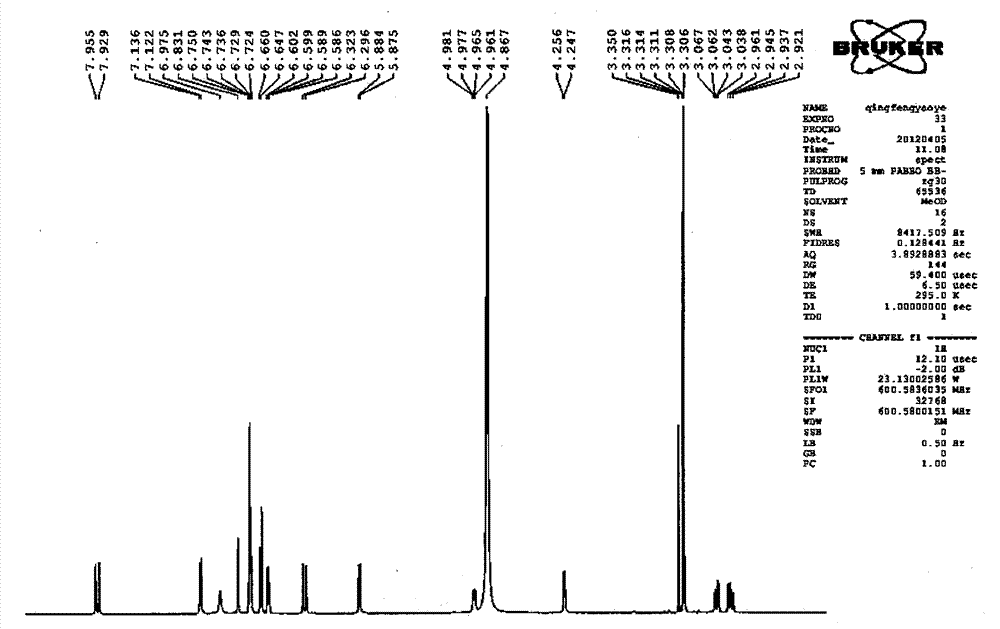
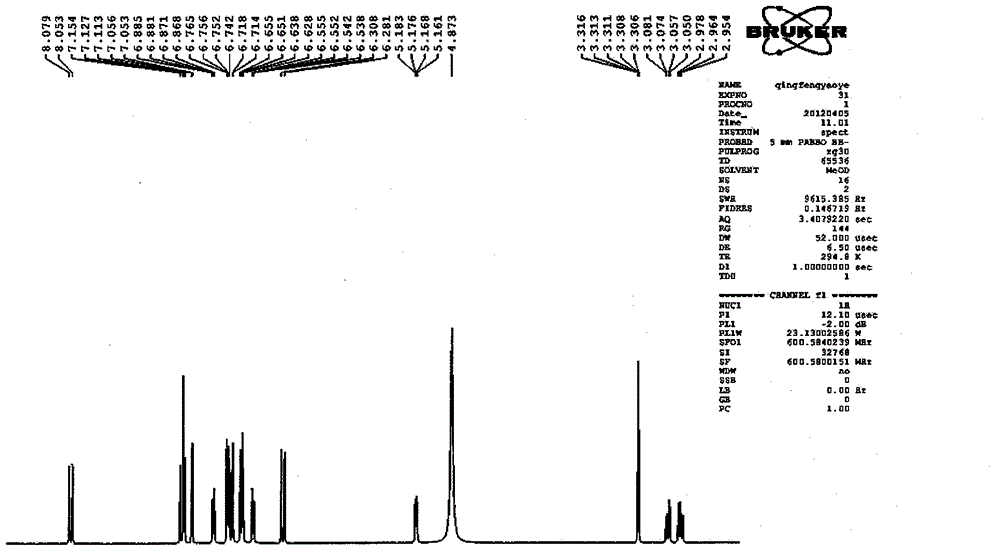
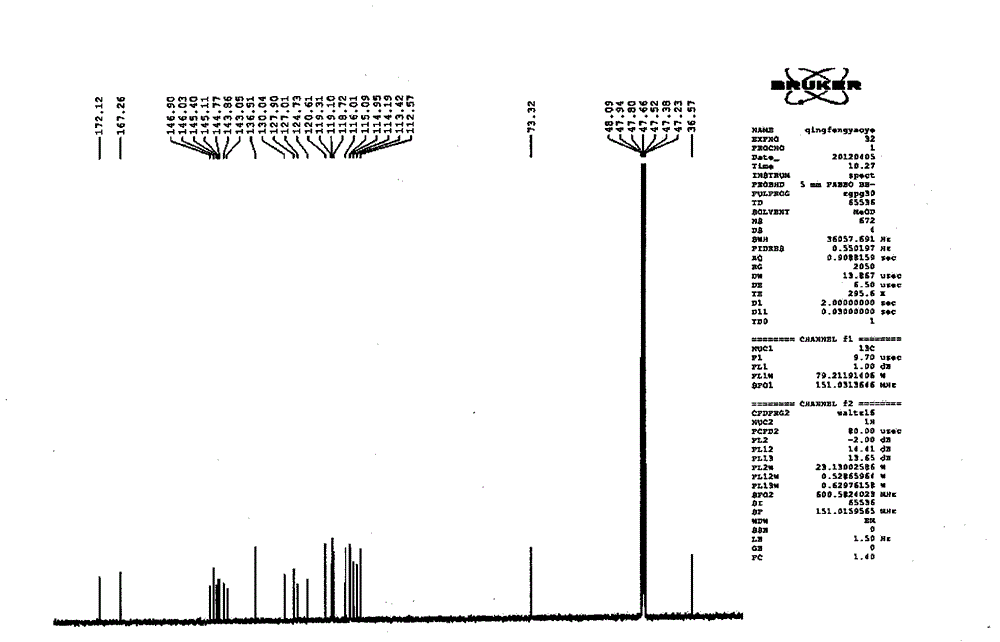
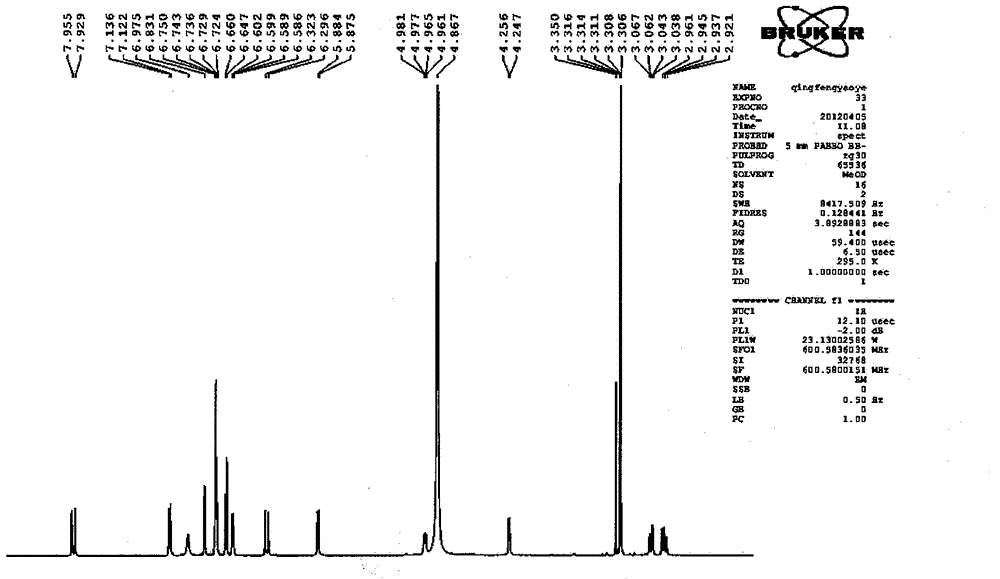
Patents
Literature
82 results about "Salvianolic acid C" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing salvianolic acid A of salvia miltiorrhiza
InactiveCN102219685AOrganic compound preparationCarboxylic acid esters preparationSalvianolic acid KGradient elution
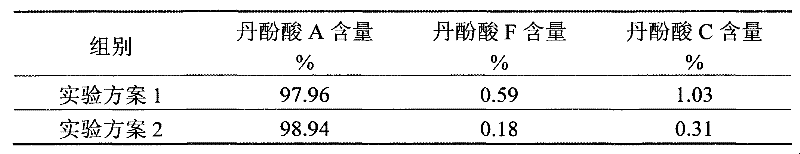
The invention discloses a method for extracting salvianolic acid A, which is characterized in that a new process method is adopted to obtain salvianolic acid A extract, the yield of the salvianolic acid A in the salvianolic acid A extract prepared with the method is more than five thousandth, thus saving a great quantity of resources. The invention further discloses a method for preparing the salvianolic acid A, which comprises the steps of adopting a medium pressure liquid phase preparation method, wherein eluent has two phases to carry out isocratic or gradient elution to obtain high-purity salvianolic acid A with related substances, such as salvianolic acid F and salvianolic acid C, with content lower than 0.5 percent. The quality of the salvianolic acid A obtained by using the method is more uniform and more stable.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
Application of salvianolic acid C in preparation of drugs for prevention and treatment of hyperuricemia
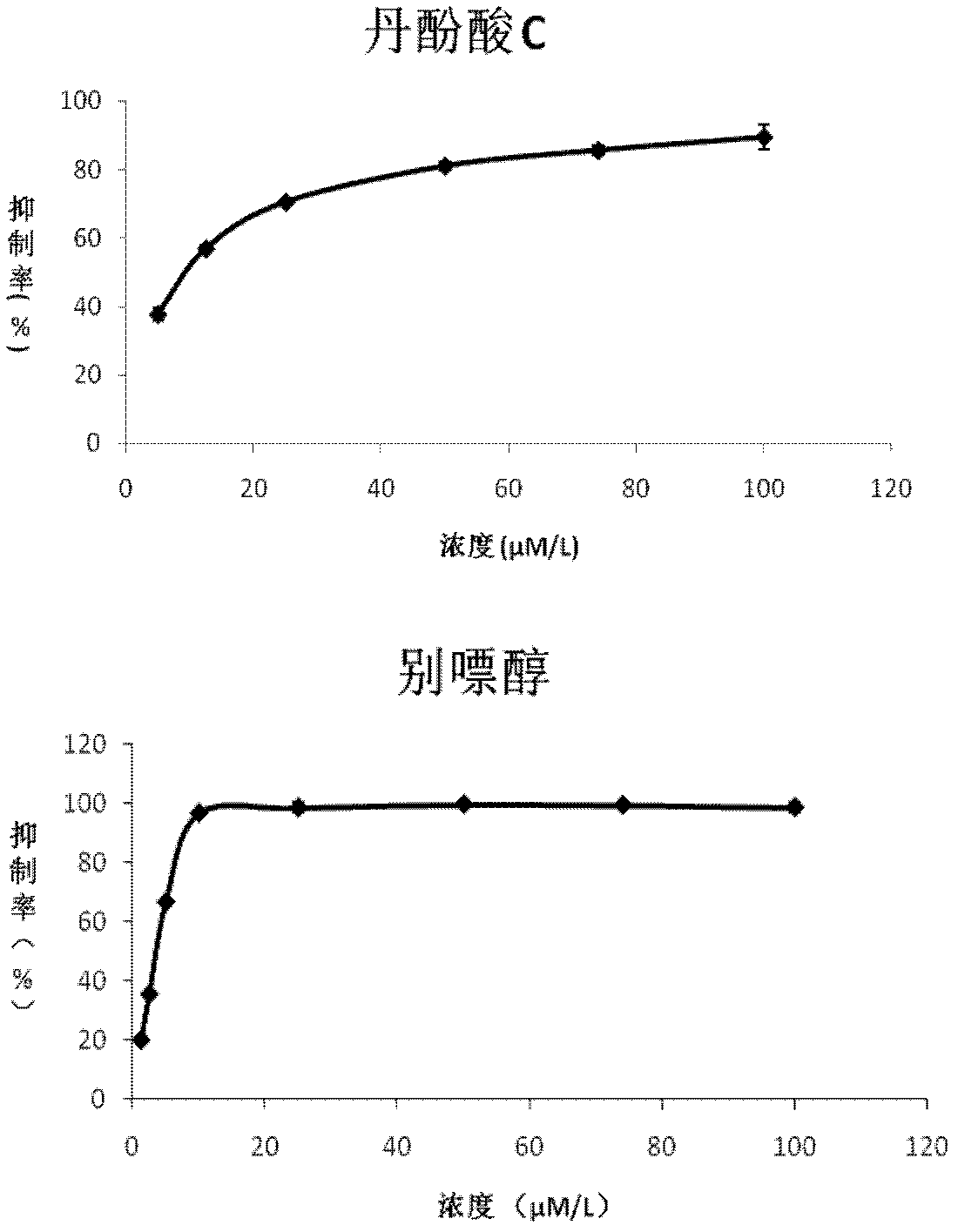
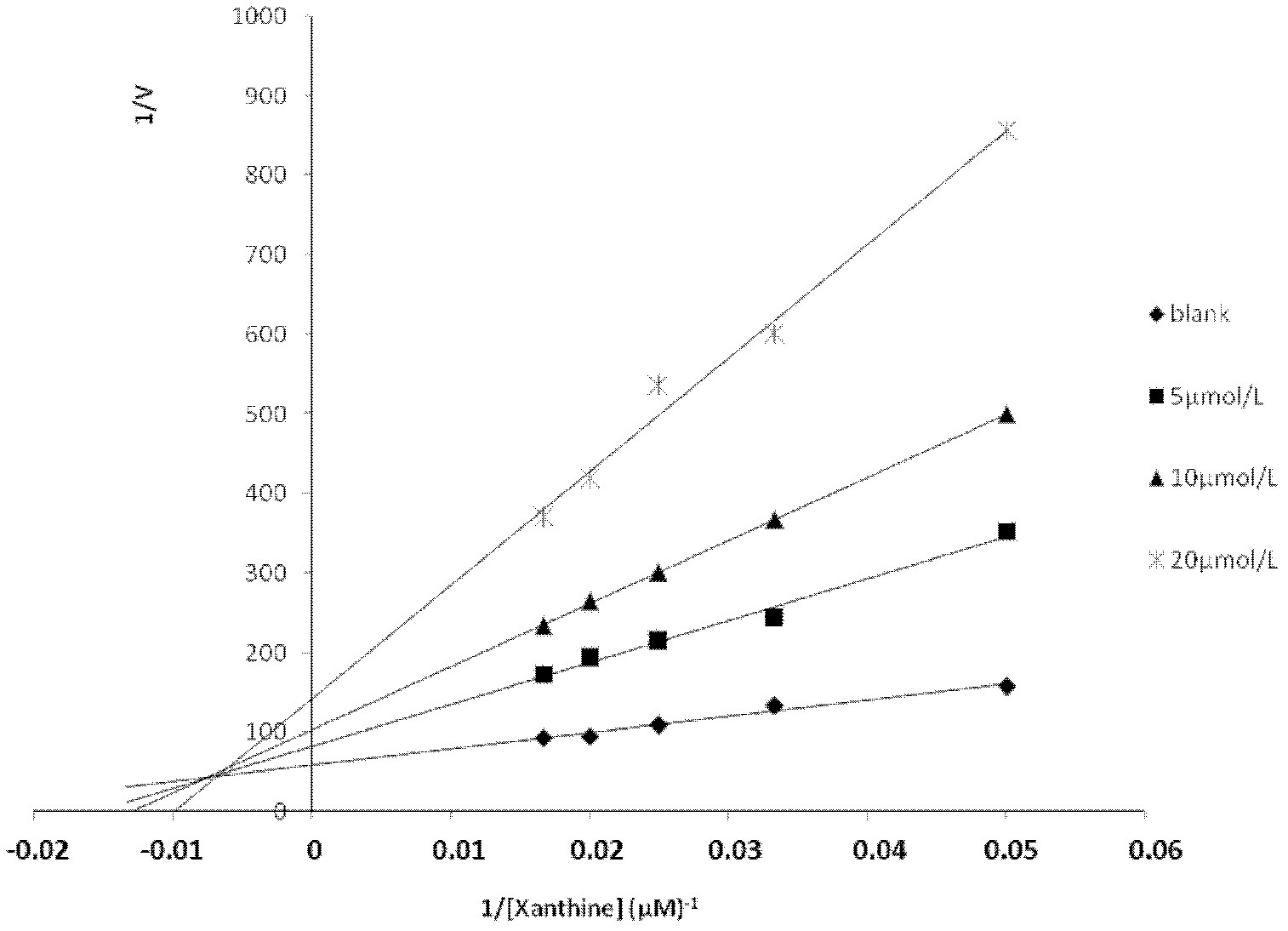
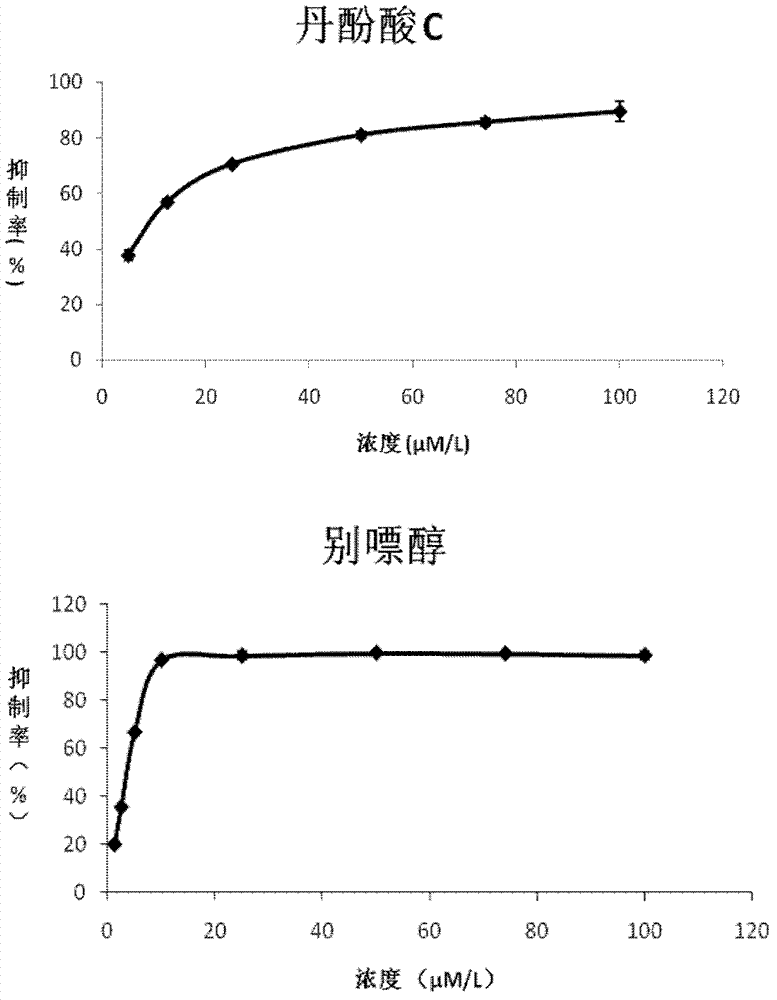
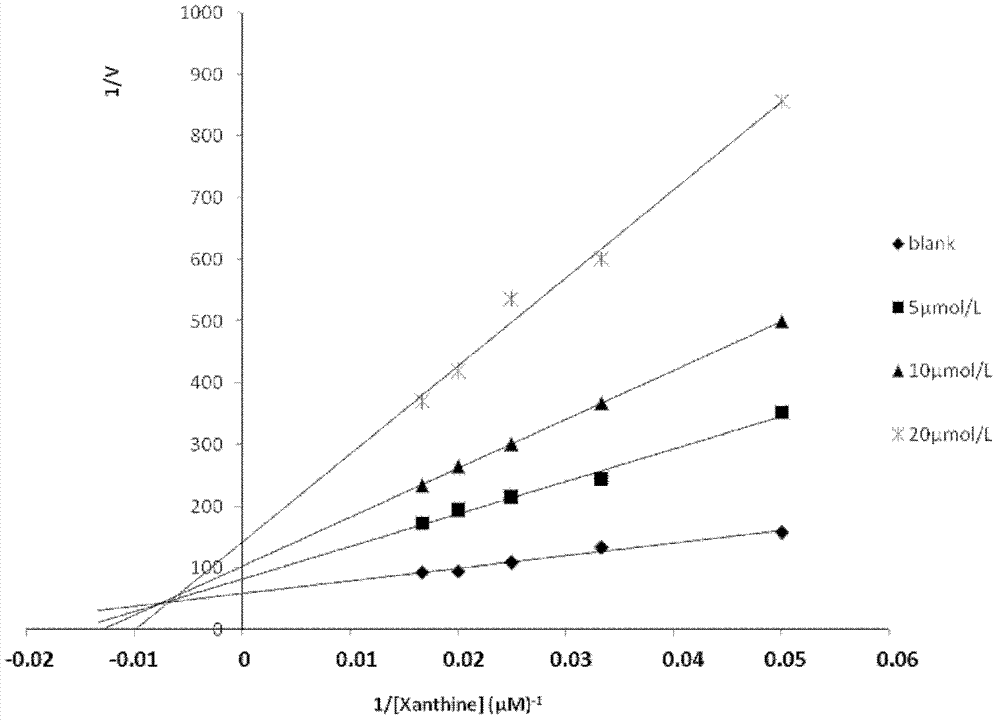
The present invention relates to the technical field of medicine, specifically to an application of salvianolic acid C in preparation of drugs for prevention and treatment of hyperuricemia. The results of the pharmacological test show that: the salvianolic acid C can be adopted as a xanthine oxidase (XO) inhibitor to prevent and treat hyperuricemia and complications of the hyperuricemia, wherein the complications caused by the hyperuricemia comprise: gout, gouty arthritis, gouty nephropathy, lithangiuria, cardiovascular diseases and other diseases.
Owner:CHINA PHARM UNIV
Application of polymer salvianolic acid in preparation of drugs used for inhibiting occurrence or development of aortic aneurysm or aortic dissection
The invention relates to application of polymer salvianolic acid in preparation of drugs used for inhibiting occurrence or development of aortic aneurysm or aortic dissection and a pharmaceutical composition comprising the polymer salvianolic acid used as a pharmaceutical active substance, related desired adjuvant and the like. The pharmaceutical composition can be used for inhibiting occurrence of aortic aneurysm or aortic dissection. The polymer salvianolic acid is dimer salvianolic acid, trimer salvianolic acid or tetramer salvianolic acid, wherein the dimer salvianolic acid is rosmarinic acid, the trimer salvianolic acid is salvianolic acid C, alkannic acid or salvianolic acid A, and the tetramer salvianolic acid is salvianolic acid B.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Application of salvianolic acid A composition in preparing medicines for improving neural function symptom after cerebral ischemia
ActiveCN103083297AGood water solubilityNot destroyedOrganic active ingredientsNervous disorderSalvianolic acid BMedicine
The invention relates to application of a salvianolic acid A composition in preparing medicines for improving neural function symptom after cerebral ischemia. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid C in preparing anti-stroke drug
InactiveCN109985033AReduce permeabilityReduce apoptosisOrganic active ingredientsCardiovascular disorderDiseaseApoptosis
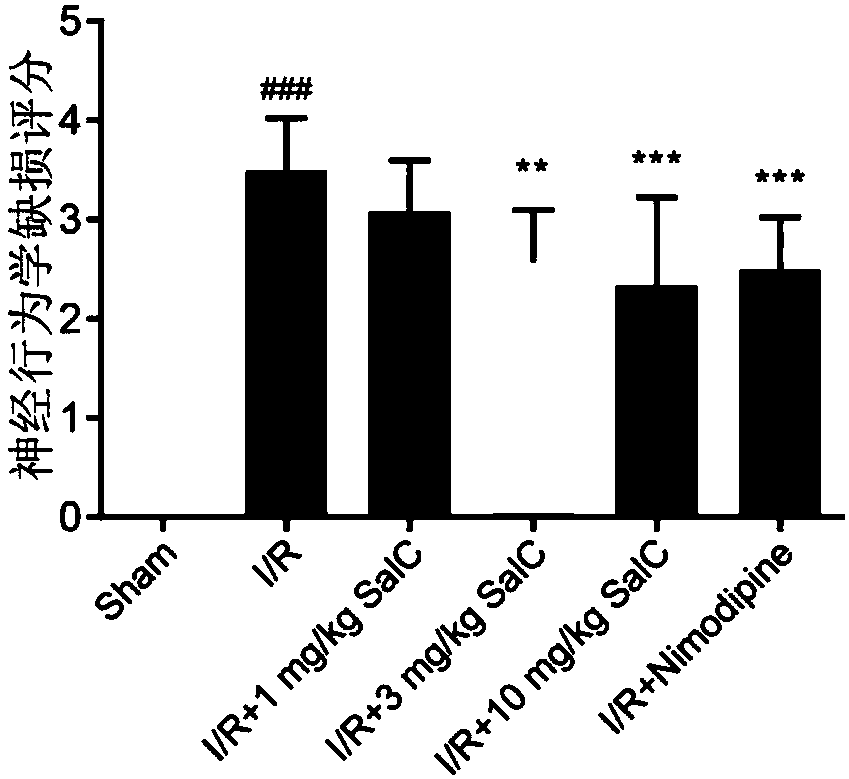
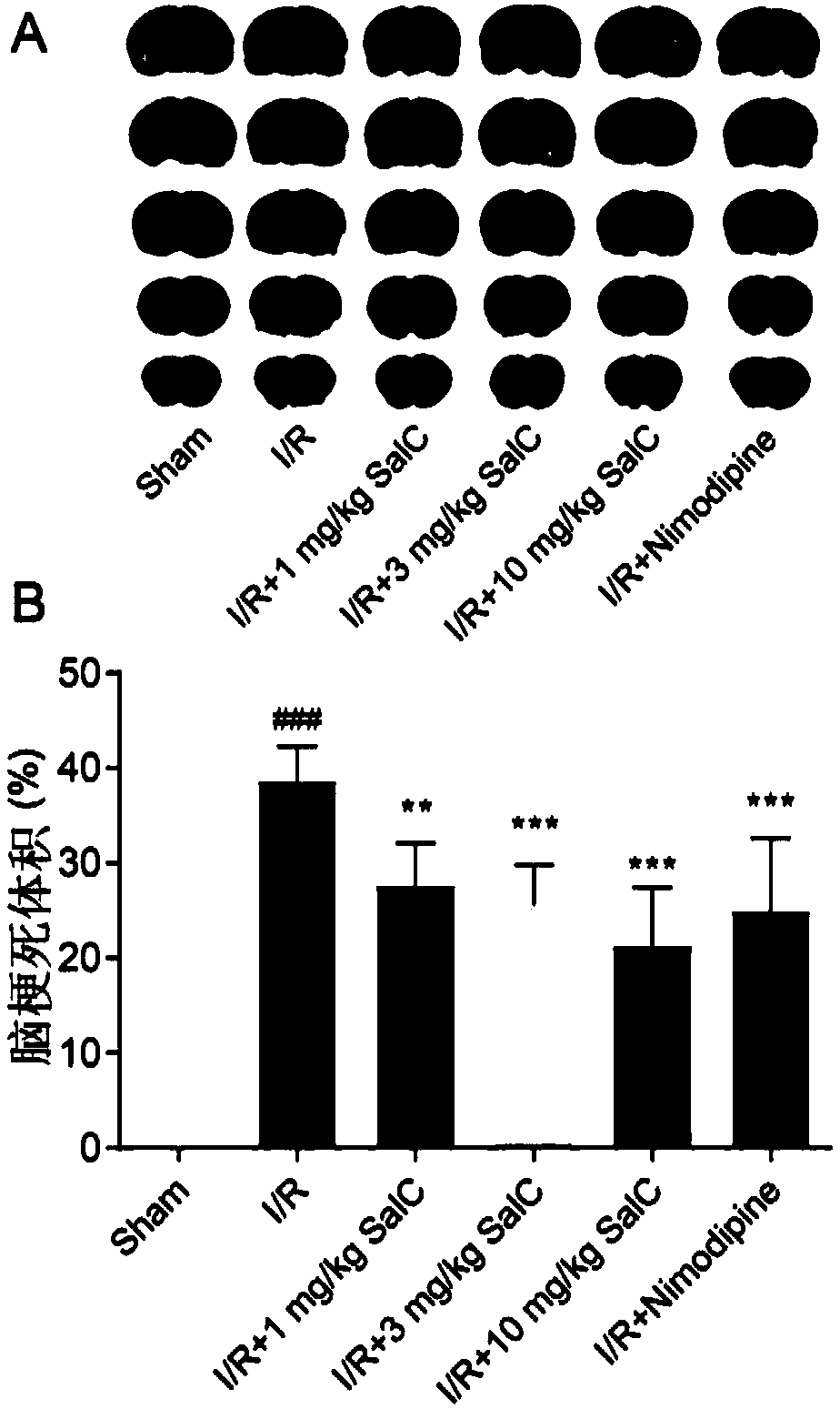
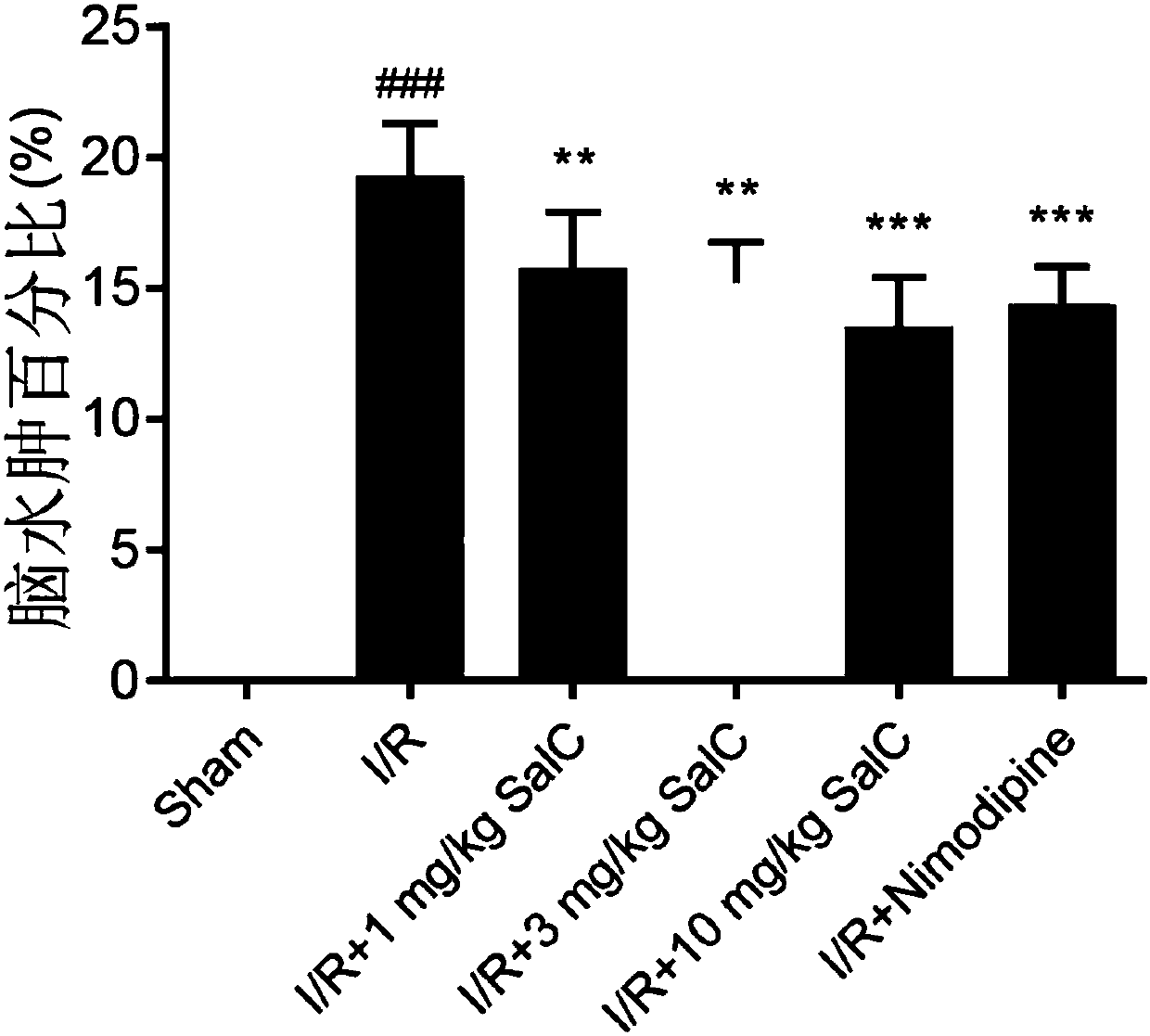
The invention discloses application of salvianolic acid C in preparing ischemic stroke resistant drug and / or healthcare products. The invention finds that the salvianolic acid C can remarkably improveneurobehavioral defect symptoms of cerebral ischemia-reperfusion rats, lower cerebral infarction volume and cerebral edema of the cerebral ischemia-reperfusion rats and reduce opening degree and permeability of rat blood brain barrier. The salvianolic acid C can be used for preparing the drug and / or the healthcare products for treating ischemic stroke, brain tissue nerve cell apoptosis can be reduced, survival rate of nerve cells can be increased, brain tissue injury degree can be lowered, and an effective solution is provided for disease prevention and treatment.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Application of salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage
The invention relates to application of a salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition for preparing medicines for preventing and/or treating cerebral thrombosis
The invention relates to application of a salvianolic acid A composition for preparing medicines for preventing and / or treating cerebral thrombosis. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition in preparing medicines for inhibiting brain tissue neuron damage or death
The invention relates to application of a salvianolic acid A composition in preparing medicines for inhibiting brain tissue neuron damage or death. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition in preparation of medicine for salvaging ischemic penumbra
The invention relates to application of a salvianolic acid A composition in preparation of a medicine for salvaging ischemic penumbra. The composition comprises the following components in parts by weight: 90 to 99% of salvianolic acid A, 0.1 to 3% of alkannic acid, 0.1 to 3% of rosmarinic acid, 0.1 to 3% of salvianolic acid B, and 0.1 to 5% salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage
ActiveCN103083302AGood water solubilityNot destroyedOrganic active ingredientsNervous disorderSalvianolic acid BMedicine
The invention relates to application of a salvianolic acid A composition in preparing medicines for protecting ischemic brain tissue damage. The composition is prepared from 94-97% of salvianolic acid A, 0.1-1.5% of alkannic acid, 0.1-1.5% of rosmarinic acid, 0.1-1.5% of salvianolic acid B and 0.1-2.0% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition for preparing medicines for preventing and/or treating cerebral thrombosis
The invention relates to application of a salvianolic acid A composition for preparing medicines for preventing and / or treating cerebral thrombosis. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
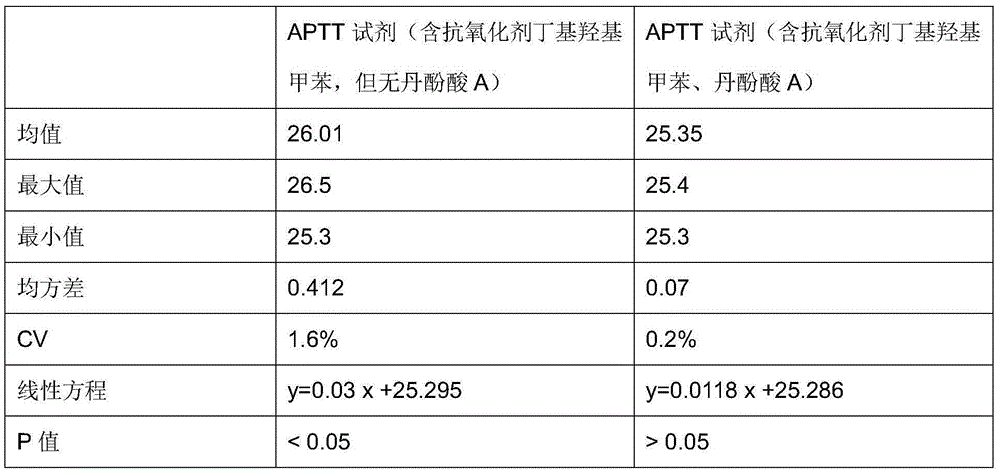
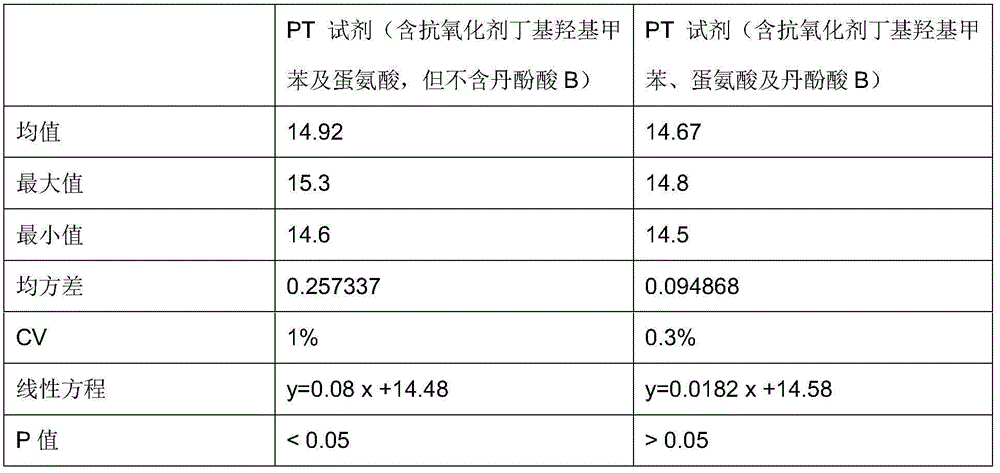
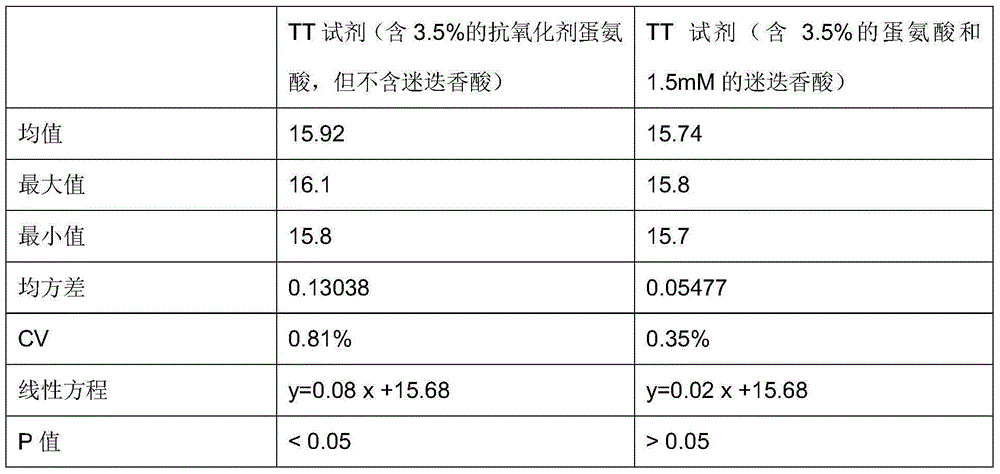
Antioxidant for coagulative reagent
ActiveCN104991079AStructural advantageStrong chelation abilityBiological testingSalvianolic acid BButylated hydroxytoluene
The invention relates to an antioxidant for a coagulative reagent. The antioxidant is salvianolic acid or rosmarinic acid, and the salvianolic acid comprises salvianolic acid A, salvianolic acid B and salvianolic acid C. The coagulative reagent comprises an APTT reagent, a PT reagent, a TT reagent, an FIB reagent and an AT-III reagent. The concentration of the salvianolic acid or rosmarinic acid in the APTT reagent is 0.5-15mM; the concentration of the salvianolic acid or rosmarinic acid in the PT reagent is 0.05-5mM; and the concentration of the salvianolic acid or rosmarinic acid in the TT reagent is 0.01-2mM. The salvianolic acid or rosmarinic acid can reduce destroys or damages of harmful metal ions and free radicals to tissue factors or thrombin in raw materials or in the process, and greatly improves the oxidation resistance of the coagulative reagent. The antioxidant can be added to other general antioxidants, such as butylated hydroxytoluene or butylated hydroxyanisole, ascorbic acid, glycine and methionine, and the antioxidants are commonly used to further improve the stability of the coagulative reagent.
Owner:QINGDAO GUGAO BIOTECH CO LTD
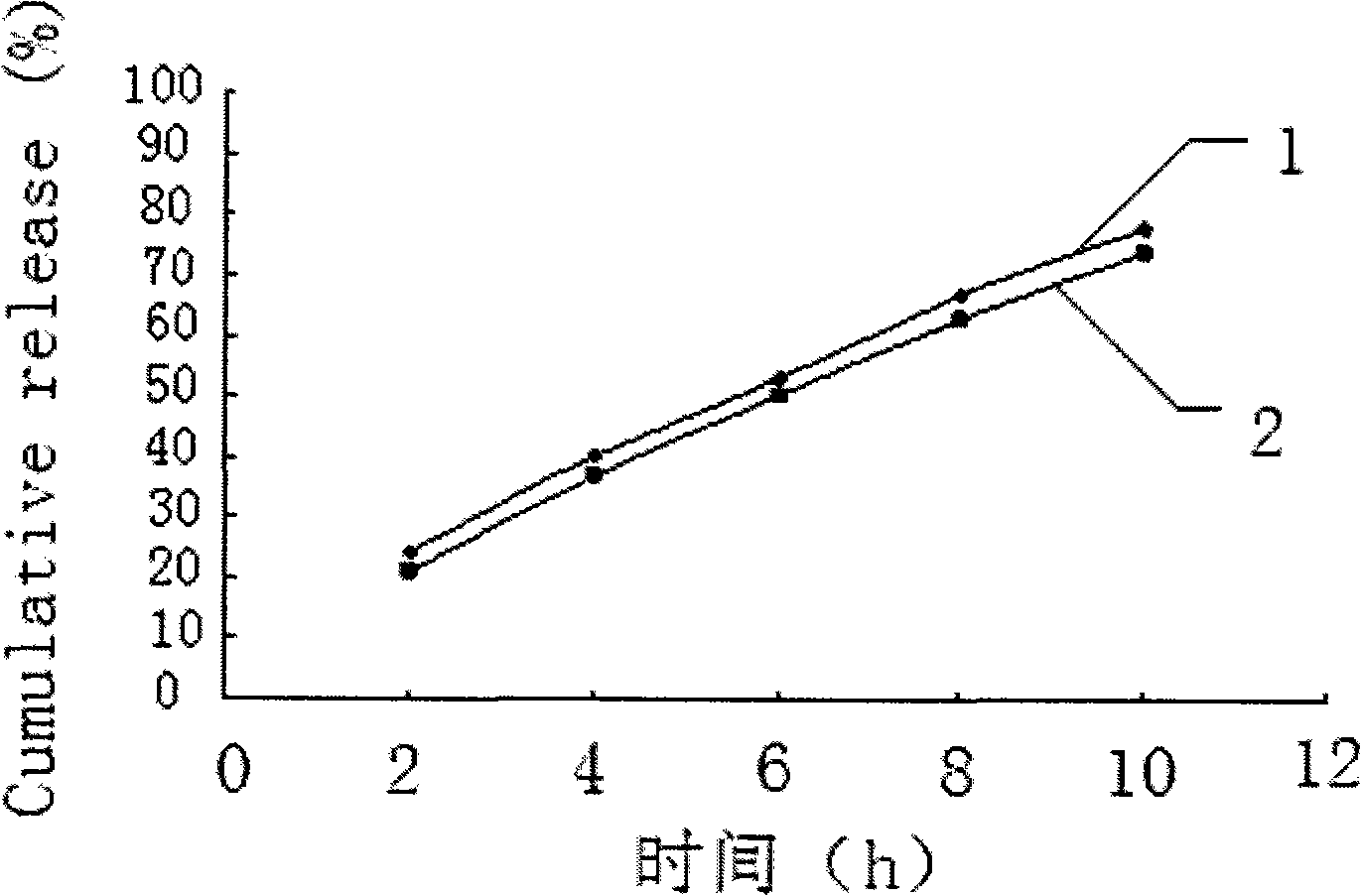
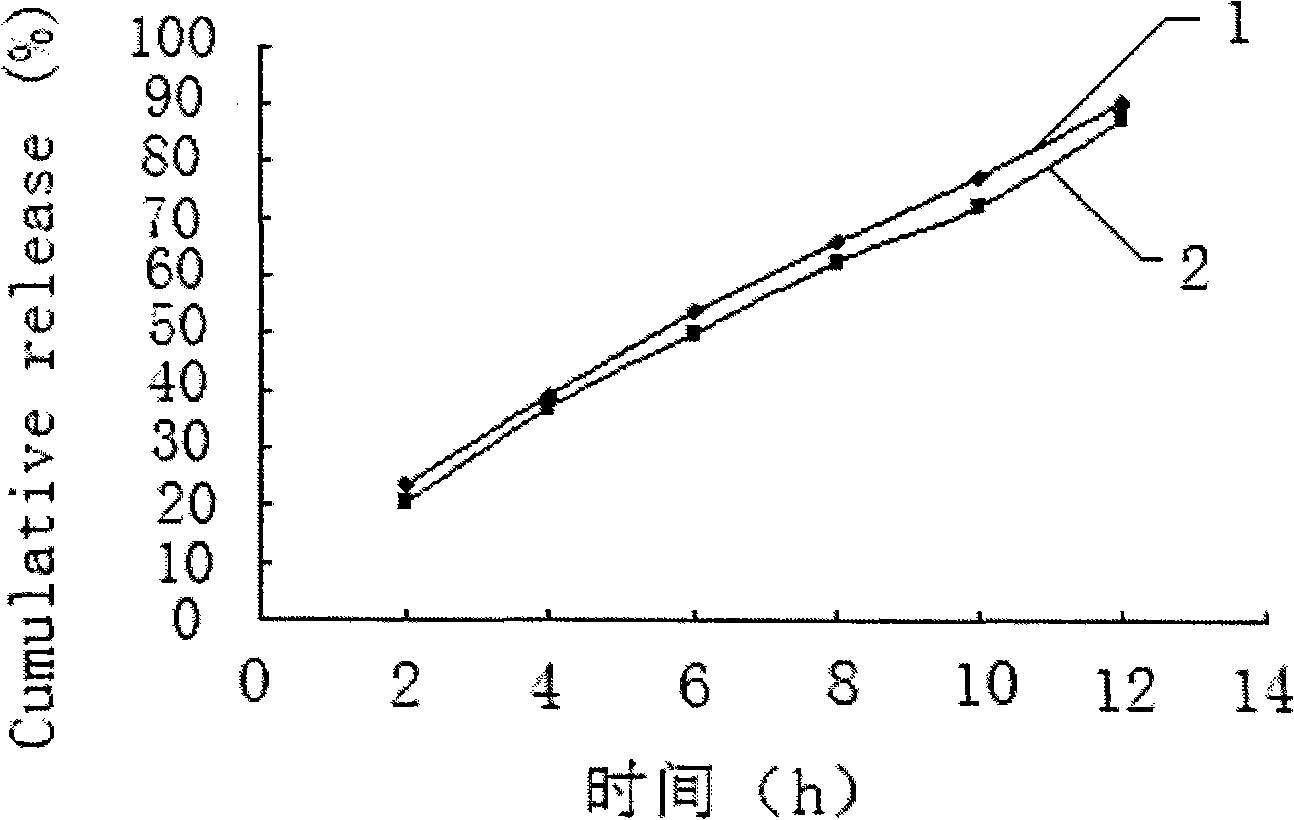
Salvianolic acid controlled porosity osmotic pump tablets and method of preparing the same
ActiveCN101278920AStable blood concentrationProlong the effective timeOrganic active ingredientsPharmaceutical delivery mechanismPorositySalvianolic acid B
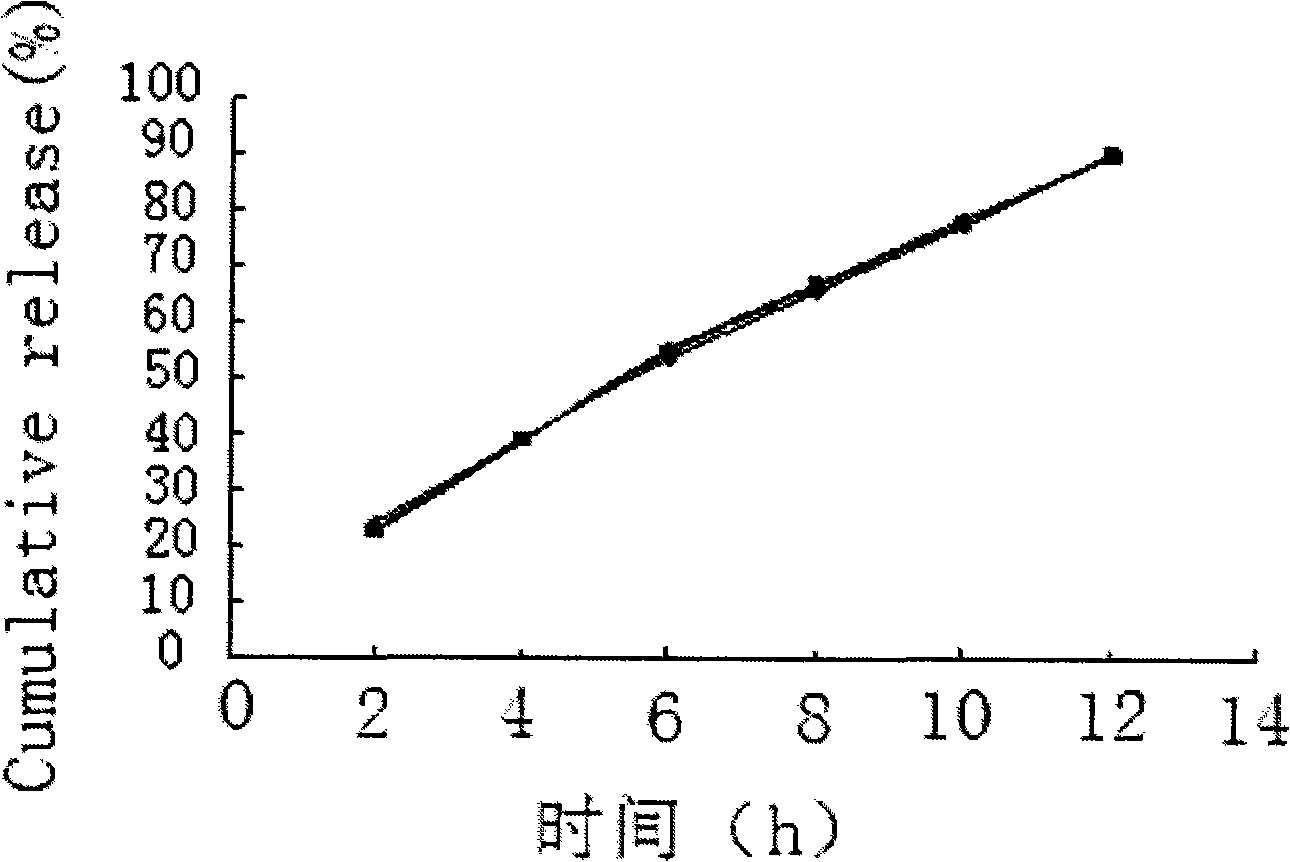
The invention discloses a salvianolic acid micropore osmotic pump controlled release tablet, comprising a tablet core and a coating; the tablet core is composed of salvianolic acid bulk drug, controlled release supplementary material, lubricant and moderate anhydrous ethanol; the weight ratio of the salvianolic acid bulk drug, the controlled release supplementary material and the lubricant is 50:150:1; the coating is composed of cellulose acetate, polyethylene glycol 400 solution and diethyl ester phthalate. In the invention, on the basis of controlling the content of 50-90% of salvianolic acid B, the content of 5-15% of tanshinol, protocatechualdehyde, salvianolic acid C, etc. in raw materials and by the optimal screening of penetrating agent, pore-forming agent, plasticizer and film thickness, an osmotic pump controlled release tablet which releases 85%-95% of a drug for 12 hours and conforms to the release characteristic of zero order kinetics is successfully prepared and used for preparing the drug for treating coronary heart disease and cerebral arteriosclerosis, which is good for the drug to form steady blood concentration in a body and lengthening the effective acting time of the drug, thus having good clinical compliance and treatment quality.
Owner:惠州市九惠药业有限公司
Application of salvianolic acid C in preparation of drugs for prevention and treatment of hyperuricemia
ActiveCN102423310BOrganic active ingredientsSkeletal disorderPurine-Xanthine OxidaseSalvianolic acid B
Owner:CHINA PHARM UNIV
Application of salvianolic acid A composition in preparing medicines for protecting cerebrovascular endothelial cells
Application of salvianolic acid A composition in preparing medicines for protecting cerebrovascular endothelial cellsThe invention relates to application of a salvianolic acid A composition for preparing medicines for protecting cerebrovascular endothelial cells. The composition is prepared from the following components in percentage by weight: 90-99% of salvianolic acid A, 0.1-3% of alkannic acid, 0.1-3% of rosmarinic acid, 0.1-3% of salvianolic acid B and 0.1-5% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvia miltiorrhiza extracts in preparing protein tyrosine phosphatase 1B inhibitor and drug for preventing and/or treating type 2 diabetes
The invention discloses application of salvia miltiorrhiza extracts in preparing a protein tyrosine phosphatase 1B inhibitor and a drug for preventing and / or treating type 2 diabetes. The salvia miltiorrhiza extracts salvianolic acid C, salvianolic acid A, danshinolic acid C or salvianolic acid A methyl ester can significantly inhibit the activity of protein tyrosine phosphatase 1B, further increase the glucose uptake rate, reduce the plasma glucose levels and prevent and / or treat the type 2 diabetes. The salvianolic acid C, the salvianolic acid A, the danshinolic acid C or the salvianolic acid A methyl ester are used as active ingredients to make a pharmaceutical preparation which can be used as the protein tyrosine phosphatase 1B inhibitor to prevent and / or treat the type 2 diabetes.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Application of salvianolic acid A freeze-dried powder to preparation of drug for protecting cerebral vascular endothelial cells
ActiveCN103142576AGood water solubilityNot destroyedPowder deliveryOrganic active ingredientsSalvianolic acid BMedicine
The invention relates to application of a salvianolic acid A freeze-dried powder to preparation of a drug for protecting cerebral vascular endothelial cells. The salvianolic acid A freeze-dried powder contains a salvianolic acid A main ingredient, a filler and an antioxidant in the following weight proportion: 20g-60g of the salvianolic acid A main ingredient, 20g-60g of the filler and the antioxidant accounting for 0.02%-0.1% of the total amount. The salvianolic acid A main ingredient comprises 94%-97% of salvianolic acid A, 0.2%-1.5% of lithospermic acid, 0.2%-1.5% of rosmarinic acid, 0.2%-1.5% of salvianolic acid B and 0.4%-2% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Salvianolic acid A injection and preparation method thereof
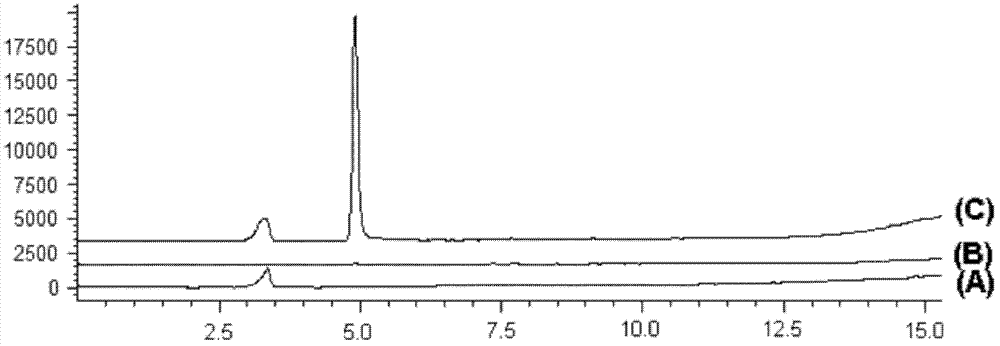
The invention discloses a salvia miltiorrhiza salvianolic acid A injection formulation and a preparation method thereof. The invention is characterized in that: the impurities in a bulk drug, namely the salvia miltiorrhiza salvianolic acid A comprise salvia miltiorrhiza salvianolic acid F and salvia miltiorrhiza salvianolic acid C; after the preparation of the formulation, the impurities in the injection formulation including the salvia miltiorrhiza salvianolic acid F and the salvia miltiorrhiza salvianolic acid C are subjected to high performance liquid chromatography measurement, the ratio between the peak area of the impurity salvia miltiorrhiza salvianolic acid F and that of the salvia miltiorrhiza salvianolic acid A of the injection formulation is more than 0 and less than 0.015, wherein, the ratio between the peak area of the impurity salvia miltiorrhiza salvianolic acid C and that of the salvia miltiorrhiza salvianolic acid A is more than 0 and less than 0.015, wherein, the ratio between the sum of the peak areas of the impurities of the injection formulation and the peak area of the salvia miltiorrhiza salvianolic acid A is more than 0 and less than 0.05; shown by pharmacological tests, the injection formation has good pharmacological actions.
Owner:CHIATAI QINGCHUNBAO PHARMA
Pharmaceutical composition for treating myocardial infarction
ActiveCN107158008AReduce toxic and side effects in vivo and in vitroReduce myocardial infarct sizeAldehyde active ingredientsCardiovascular disorderSide effectSalvianolic acid B
The invention discloses a pharmaceutical composition for treating myocardial infarction. The pharmaceutical composition comprises a tanshinone compound shown in a formula (I) and a phenolic acid compound containing one or more substituent shown in a formula (II), preferably, comprises tanshinone I and tanshinol or tanshinone IIA and salvianolic acid B or dihydrotanshinone I and protocatechualdehyde or cryptotanshinone and salvianolic acid C. According to the invention, the phenolic acid compound containing the specific substituent and the tanshinone compound having a specific structure are prepared to obtain the pharmaceutical composition. The pharmaceutical composition can effectively reduce the myocardial infarction area of mice with acute myocardial infarction, and has low toxic and side effects; the phenolic acid compound in the composition can obviously reduce the in vitro and vivo toxic and side effects of the tanshinone compound, and can enhance the cardiac muscle protection effect of the tanshinone compound on the mice with acute myocardial infarction.
Owner:CHINA PHARM UNIV
Application of salvianolic acid A freeze-dried powder to preparation of drug for inhibiting neuronal damage or death of brain tissue
The invention relates to application of a salvianolic acid A freeze-dried powder to preparation of a drug for inhibiting neuronal damage or death of brain tissue. The salvianolic acid A freeze-dried powder contains a salvianolic acid A main ingredient, a filler and an antioxidant in the following weight proportion: 20g-60g of the salvianolic acid A main ingredient, 20g-60g of the filler and the antioxidant accounting for 0.02%-0.1% of the total amount. The salvianolic acid A main ingredient comprises 94%-97% of salvianolic acid A, 0.2%-1.5% of lithospermic acid, 0.2%-1.5% of rosmarinic acid, 0.2%-1.5% of salvianolic acid B and 0.4%-2% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
GPR35 receptor stimulant in salviae miltiorrhizae, inhibitor of Ca2+-ATPase and application
InactiveCN105521016AExpand the scope of clinical applicationNervous disorderAntipyreticATPaseSalvianolic acid B
The invention relates to the discovering of the action targets of traditional Chinese medicine salviae miltiorrhizae and widening of clinical application range, in particular to discovering of the new action targets of phenolic acid compounds in the salviae miltiorrhizae and new clinical application of the phenolic acid compounds. The phenolic acid compounds include similar-structured compounds such as protocatechuic aldehyde, tanshinol, caffeic acid, alkannic acid, salvianolic acid A, salvianolic acid B, salvianolic acid C and salvianolic acid D, the derivatives of the similar-structured compounds and the pharmaceutically-acceptable salt-forming compounds of the similar-structured compounds. In vitro cell experiments show that the compounds act on the orphan receptor GPR35 and current researches show that the orphan receptor GPR35 is related to diseases such as cardiac failure, hypertension, coronary heart disease, metabolic syndrome, asthma, pain, inflammation and cancer, so that the clinical application range of the compounds can be widened. In addition, the fact that the compounds have a double-target effect is discovered, and certain guidance is provided for the new clinical application.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Application of salvianolic acid A freeze-dried injection in preparing drug for rescuing ischemic penumbra
ActiveCN103432110AGood water solubilityNot destroyedOrganic active ingredientsPowder deliverySalvianolic acid BMedicine
The invention relates to an application of a salvianolic acid A freeze-dried injection in preparing a drug for rescuing ischemic penumbra. The salvianolic acid A freeze-dried injection comprises a salvianolic acid A-containing main raw material, a filling agent and an antioxidant. The freeze-dried injection is prepared by the following components by weight: 20 g-60 g of the salvianolic acid A-containing main raw material, 20 g-60 g of the filling agent and the antioxidant accounting for 0.02%-0.1% of a total weight. The salvianolic acid A-containing main raw material comprises 94%-97% of salvianolic acid A, 0.2%-1.5% of alkannic acid, 0.2%-1.5% of rosmarinic acid, 0.2%-1.5% of salvianolic acid B and 0.4%-2.0% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
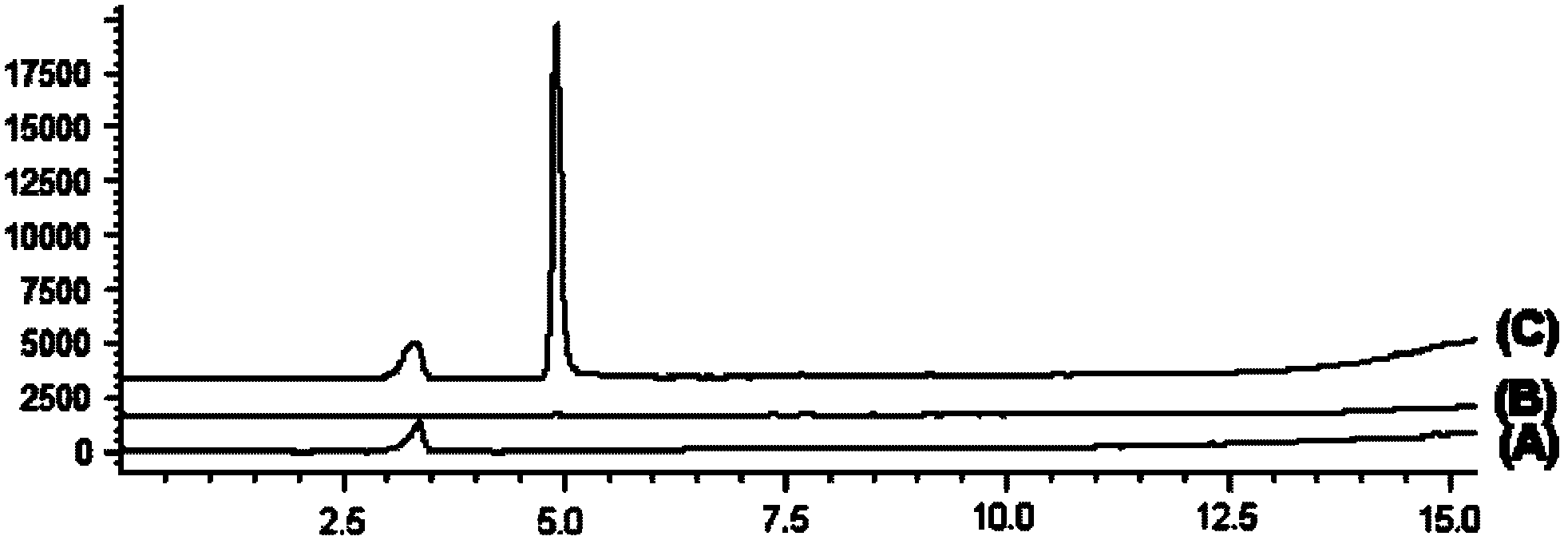
Chan tablet liquid chromatography-mass spectrometry determination method
PendingCN114113403AComponent separationAgainst vector-borne diseasesSalvianolic acid BO-Phosphoric Acid
The invention relates to a liquid chromatography-mass spectrometry determination method for a Holdan tablet. The method comprises the following steps: mixing psoralen glycoside, isopsoralen glycoside, hyperoside, quercetin 3-O-glucuronide, isoquercitrin, sennoside B, astragalus smicus glycoside, sennoside A, rosmarinic acid, alkannic acid, salvianolic acid B, nuciferine, salvianolic acid A, psoralen, isopsoralen, salvianolic acid C, rhein, neopsoralen isoflavone, psoralen and cryptotanshinone, and uniformly stirring to obtain a mixture; the method comprises the following steps of: preparing reference substance solutions A and B and a test sample solution by taking a sample as a reference substance and bakuchiol as a reference substance, injecting the reference substance solutions A and B and the test sample solution into a liquid chromatograph-mass spectrometer, obtaining mass spectrograms when A is in a positive ion mode and B is in a negative ion mode, and calculating the content of the effective components in the test sample according to the mass spectrograms under the chromatographic conditions that an Agilent C18 chromatographic column (4.6 mm * 250 mm, 5 [mu] m), the flow rate is 0.4 mL.min <-1 > and the sample injection volume is 2 [mu] L. The column temperature is 30 DEG C, and a 0.2% phosphoric acid aqueous solution (A)-acetonitrile (B) is used as a mobile phase for gradient elution.
Owner:NANCHANG JISHUN PHARMA CO LTD
Salvianolic acid A dropping pill and application thereof to medicine preparation
ActiveCN103142516AGood water solubilityNot destroyedOrganic active ingredientsOrganic chemistryWaxDisease
The invention relates to application of a salvianolic acid A dropping pill to preparation of medicine for preventing and / or treating ischemic cerebrovascular disease. The salvianolic acid A dropping pill contains by weight 5%-60% of a salvianolic acid A composition and 95%-40% of a substrate. The salvianolic acid A composition comprises salvianolic acid A more than 93% and less than 100%, and other 4 ingredients including 0.1%-2% of alkannic acid, 0.1%-2% of rosmarinic acid, 0.1%-2% of salvianolic acid B and 0.1%-2% of salvianolic acid C. The substrate is one or more selected from PEG 4000, PEG 6000, PEG 8000, gelatin, stearic acid, glycerol monostearate, insect wax and hydrogenated vegetable oil.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Salvianolic acid A dropping pill and application thereof to medicine preparation
ActiveCN103142517ANot destroyedBest extraction processOrganic active ingredientsNervous disorderWaxSalvianolic acid B
The invention relates to a salvianolic acid A dropping pill. The salvianolic acid A dropping pill contains by weight 5%-60% of a salvianolic acid A composition and 95%-40% of a substrate. The salvianolic acid A composition comprises salvianolic acid A more than 93% and less than 100%, and other 4 ingredients including 0.1%-2% of alkannic acid, 0.1%-2% of rosmarinic acid, 0.1%-2% of salvianolic acid B and 0.1%-2% of salvianolic acid C. The substrate is one or more selected from PEG 4000, PEG 6000, PEG 8000, gelatin, stearic acid, glycerol monostearate, insect wax and hydrogenated vegetable oil. The invention also discloses application of the salvianolic acid A dropping pill to preparation of medicine for preventing and / or treating ischemic cerebrovascular disease.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Purpose of salvianolic acid A freeze-dried powder injection for preparing medicine for saving ischemic penumbra
ActiveCN103083259AGood water solubilityNot destroyedPowder deliveryOrganic active ingredientsVitamin CSalvianolic acid B
The invention relates to a purpose of a salvianolic acid A freeze-dried powder injection for preparing a medicine for saving ischemic penumbra. The salvianolic acid A freeze-dried powder injection comprises the following components in weight ratio: 10-80g of main ingredient containing salvianolic acid A, 10-80g of filler, and 0.01-0.2% of antioxidizer, wherein the filler is any one or more selected from mannitol, glucose and lactose; the antioxidizer is any one or more selected from vitamin C, thiourea, sodium hydrogen sulphite and sodium pyrosulfite; the content of the salvianolic acid A in the main ingredient containing salvianolic acid A is more than 93% and less than 100%; and the salvianolic acid A freeze-dried powder injection further comprises other four components: 0.1-2% of alkannic acid, 0.1-2% of rosmarinic acid, 0.1-2% of salvianolic acid B, and 0.1-2% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A freeze-dried powder injection for preparing medicines for preventing and/or treating cerebral thrombosis
ActiveCN103083256AGood water solubilityNot destroyedPowder deliveryOrganic active ingredientsVitamin CSalvianolic acid B
The invention relates to application of a salvianolic acid A freeze-dried powder injection for preparing medicines for preventing and / or treating cerebral thrombosis. The salvianolic acid A freeze-dried powder injection comprises 10-80g of main raw material containing salvianolic acid A, 10-80g of filler and 0.01-0.2 wt% of antioxidant, wherein the filler is any one or more of mannitol, glucose and lactose; the antioxidant is selected from any one or more of vitamin C, thiocarbamide, sodium bisulfite and sodium metabisulfite; and the main raw material containing salvianolic acid A comprises greater than 93% and less than 100% of salvianolic acid A, 0.1-2% of alkannic acid, 0.1-2% of rosmarinic acid, 0.1-2% of salvianolic acid B and 0.1-2% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Salvianolic acid A freeze-dried powder and application thereof to medicine preparation
ActiveCN103142579AGood water solubilityNot destroyedOrganic active ingredientsPowder deliveryVitamin CSalvianolic acid B
The invention relates to application of a salvianolic acid A freeze-dried powder to preparation of medicine for preventing and treating ischemic heart disease. The salvianolic acid A freeze-dried powder contains a salvianolic acid A main ingredient, a filler and an antioxidant in the following weight proportion: 10g-80g of the salvianolic acid A main ingredient, 10g-80g of the filler and the antioxidant accounting for 0.01%-0.2% of the total amount. The filler is one or more selected from mannitol, glucose and lactose. The antioxidant is one or more selected from vitamin C, thiourea, sodium bisulfite and sodium pyrosulfite. The salvianolic acid A main ingredient comprises salvianolic acid A more than 93% and less than 100%, and other four components: 0.1%-2% of alkannic acid, 0.1%-2% of rosmarinic acid, 0.1%-2% of salvianolic acid B, and 0.1%-2% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of salvianolic acid A composition in preparing medicines for protecting cerebrovascular endothelial cells
ActiveCN103083300AGood water solubilityNot destroyedOrganic active ingredientsNervous disorderSalvianolic acid BMedicine
The invention relates to application of a salvianolic acid A composition for preparing medicines for protecting cerebrovascular endothelial cells. The composition comprises 94-97% of salvianolic acid A, 0.1-1.5% of alkannic acid, 0.1-1.5% of rosmarinic acid, 0.1-1.5% of salvianolic acid B and 0.1-2.0% of salvianolic acid C.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Salvianolic acid A capsule and application thereof to medicine preparation
ActiveCN103142542ANot destroyedBest extraction processOrganic active ingredientsNervous disorderDiseaseSucrose
The invention relates to a salvianolic acid A capsule comprising by weight 5%-60% of a salvianolic acid A composition, 94.9%-39.5% of a filler and 0.1%-0.5% of a lubricant. The salvianolic acid A composition comprises salvianolic acid A more than 93% and less than 100%, and other 4 ingredients including 0.1%-2% of alkannic acid, 0.1%-2% of rosmarinic acid, 0.1%-2% of salvianolic acid B, 0.1%-2% of salvianolic acid C. The filler is one or more selected from starch, dextrin, sucrose, mannitol, microcrystalline cellulose, pregelatinized starch and calcium sulfate. The lubricant is one or more selected from magnesium stearate, talcum powder, superfine silica powder and polyethylene glycol. The invention also discloses application of the salvianolic acid A capsule to preparation of medicine for preventing and / or treating ischemic cerebrovascular disease.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com