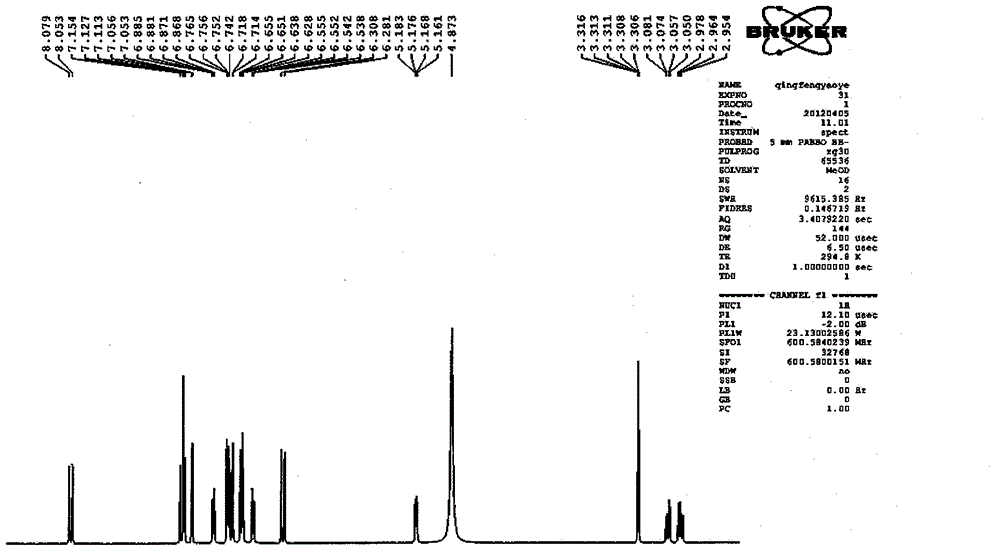
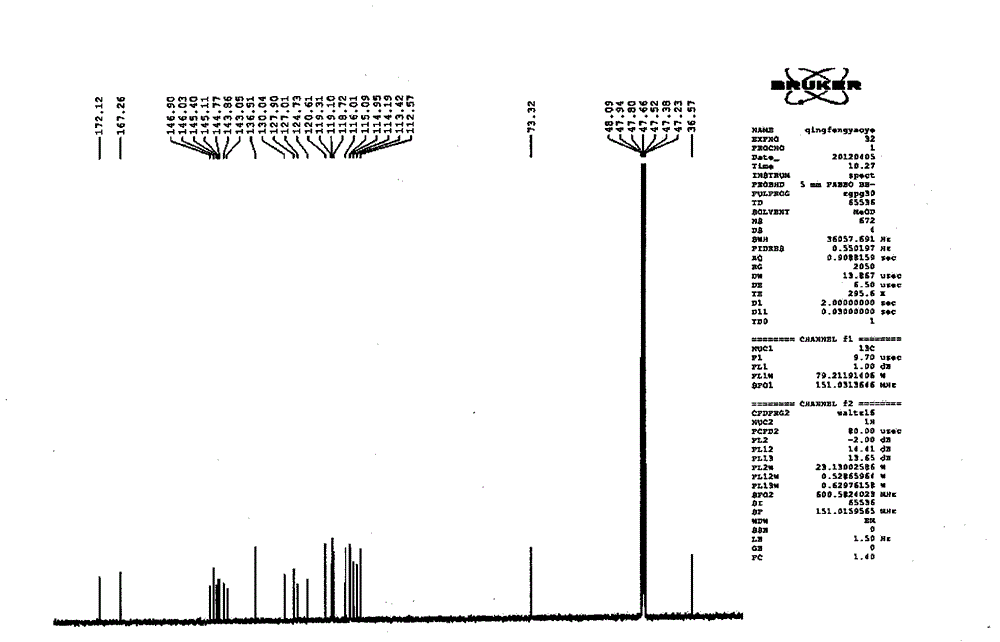
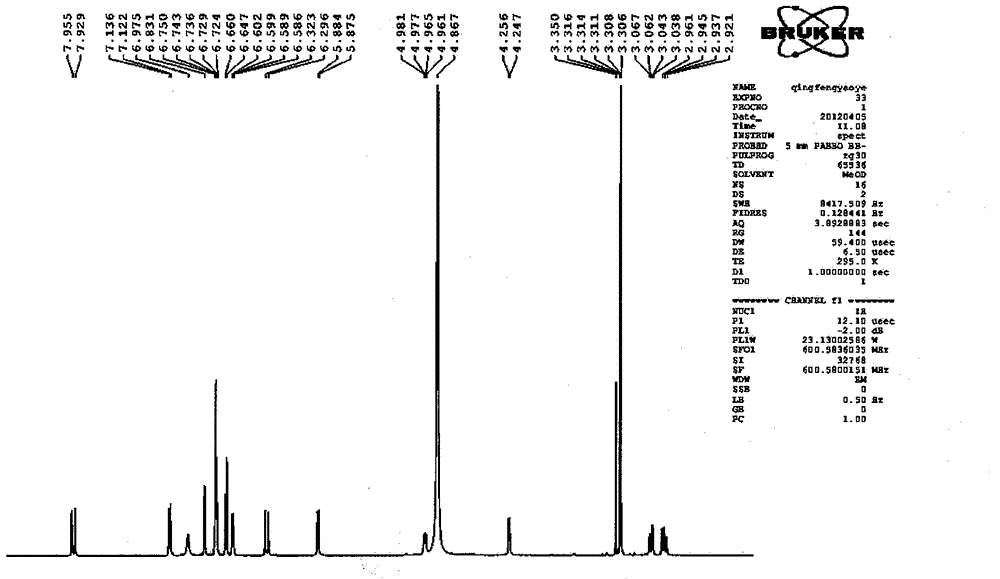
Salvianolic acid A capsule and application thereof to medicine preparation
A kind of technology of salvianolic acid and capsules, applied in the field of salvianolic acid A capsules and preparation of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Take Danshen medicinal material, crush it into 4-mesh granules, add 7 times the amount of 92°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to relative Density 1.0-1.30 (60°C), add ethanol to make the alcohol content 70%, let it stand, filter, and recover ethanol from the filtrate under reduced pressure and concentrate until there is no alcohol smell; add water to dilute to 20mg of salvianolic acid B per 1ml, for aqueous solution 10% sodium hydroxide to adjust pH to 5.5, add 0.5% ZnCl 2As a catalyst, heat and transform at 120°C for 4 hours, adjust the pH value of the transformation liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and perform HPD-100 macroporous resin column chromatography For separation, the ratio of the loading amount of sal...
Embodiment 2
[0136] Take the Danshen medicinal material, crush it into particles with a diameter of about 2mm, add 9 times the amount of 95°C water each time, warm soak and extract 3 times, and stir at a speed of 30 rpm, and warm soak and extract for 2.5 hours each time; the extract is concentrated under reduced pressure to Relative density 1.0~1.30 (60°C), add ethanol to make the alcohol content 70%, let it stand, filter, the filtrate recovers ethanol under reduced pressure and concentrates until there is no alcohol smell; add water to dilute to contain salvianolic acid B15mg per 1ml, aqueous solution Adjust pH to 4.5 with 10% NaOH, add 0.5% ZnCl 2 As a catalyst, heat conversion at 120°C (0.01MPa~0.20MPa pressure) for 4 hours, adjust the pH value of the conversion liquid to 2.8 with 15% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 5 mg of salvianolic acid A per 1 ml, and pass through HPD -100 macroporous resin column chromatography separation,...
Embodiment 3
[0139] Take Salvia miltiorrhiza, crush it into particles with a diameter of about 2mm, add 7 times the amount of 90°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, and warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to Relative density 1.0~1.30 (60°C), add ethanol to make the alcohol content 70%, let it stand, filter, the filtrate recovers ethanol under reduced pressure and concentrates until there is no alcohol smell; add water to dilute to contain salvianolic acid B20mg per 1ml, aqueous solution Adjust pH to 5.5 with 10% NaOH, add 0.5% ZnCl 2 As a catalyst, heat conversion at 120°C (0.01MPa~0.20MPa pressure) for 4 hours, adjust the pH value of the conversion liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and pass through HPD -100 macroporous resin column chromatography separation, the ratio of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| angle of repose | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com