Application of salvianolic acid A freeze-dried powder to preparation of drug for inhibiting neuronal damage or death of brain tissue
A technology of neuron damage and freeze-dried powder injection, which is applied in the direction of carboxylate preparation, freeze-dried delivery, drug combination, etc., can solve the problems of instability of injections and the inability to guarantee the pharmacological effects of salvianolic acid A, so as to improve the transformation rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
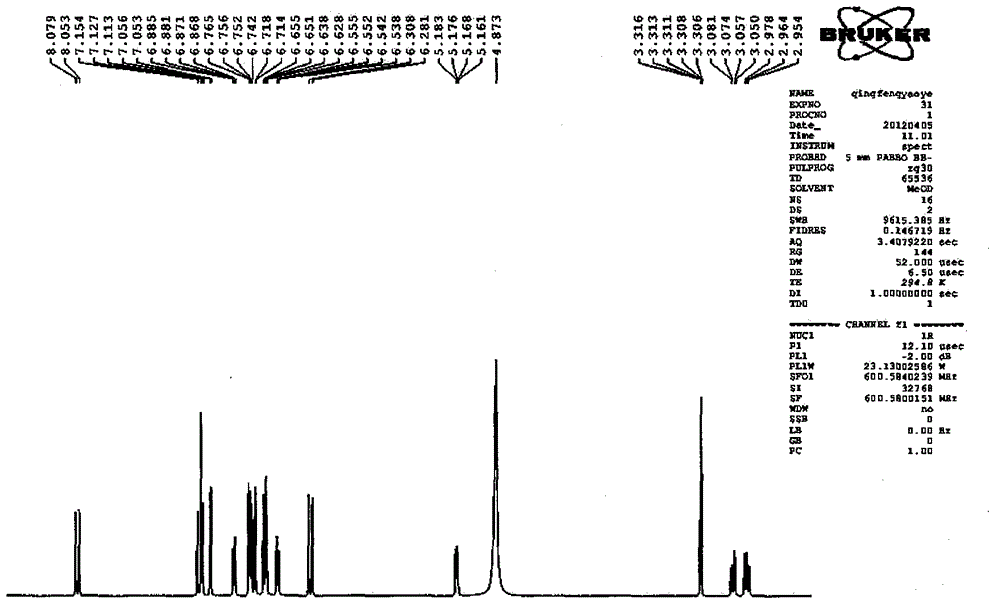
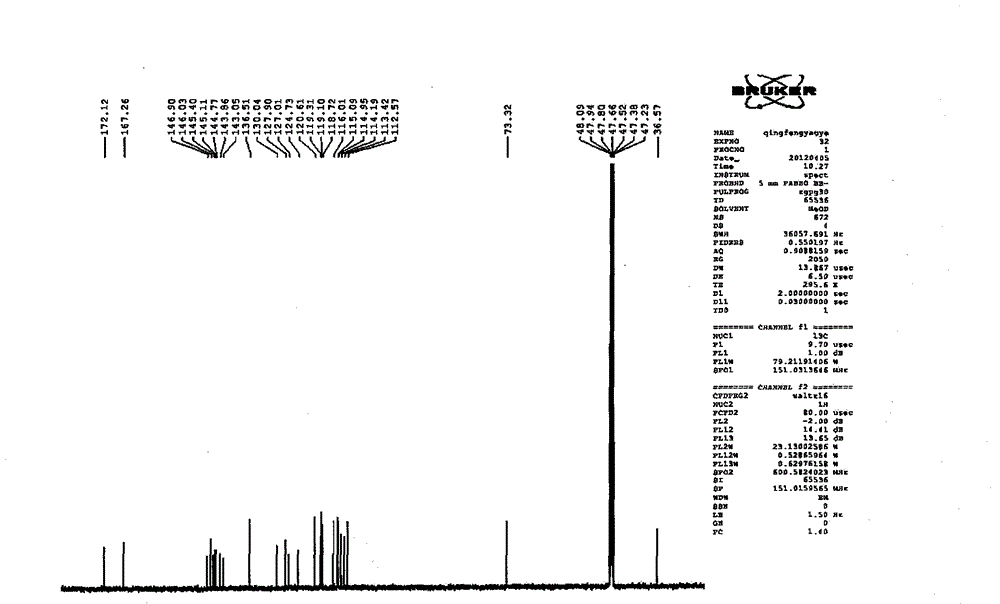
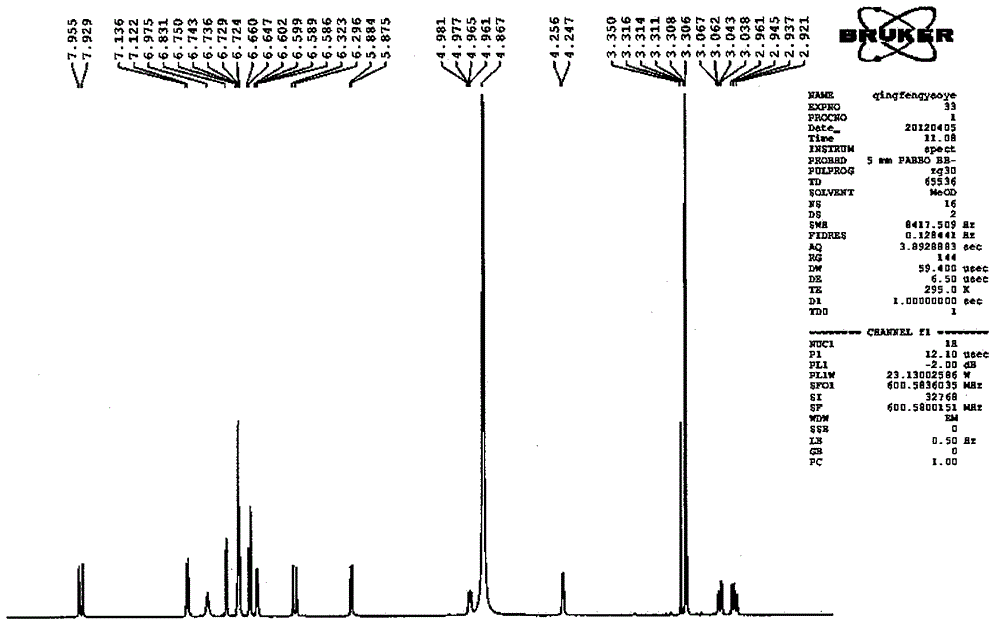
[0099] Take Danshen medicinal material, crush it into 6-mesh granules, add 7 times the amount of 92°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, and warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to relative Density 1.20 (60°C), add ethanol to make the alcohol content at 70%, let it stand, filter, the filtrate is decompressed to recover ethanol and concentrate until it has no alcohol smell; add water to dilute to contain salvianolic acid B20mg per 1ml, use 10% for aqueous solution Adjust the pH to 4.0 with sodium hydroxide, add 0.5% ZnCl 2 As a catalyst, heat and transform at 120°C for 4 hours, adjust the pH value of the transformation liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and perform HPD-100 macroporous resin column chromatography For separation, the ratio of the loading amount of ...
Embodiment 2
[0102] Take Salvia miltiorrhiza, crush it into particles with a diameter of about 2mm, add 7 times the amount of 90°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, and warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to Relative density 1.16 (60°C), add ethanol to make the alcohol content 70%, let stand, filter, the filtrate recovers ethanol under reduced pressure and concentrates until there is no alcohol smell; add water to dilute to contain salvianolic acid B20mg per 1ml, and use 10 mg of salvianolic acid B in the aqueous solution % NaOH to adjust the pH to 3.5, add 0.5% ZnCl 2 As a catalyst, heat and transform at 120°C for 4 hours, adjust the pH value of the transformation liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and perform HPD-100 macroporous resin column chromatography For separation...
Embodiment 3
[0105] Take Salvia miltiorrhiza, cut into decoction pieces, add 8 times the amount each time, soak in water at 85°C for 3 times, stir at a speed of 20 rpm, and extract with warm soaking for 2.5 hours each time; the extract is concentrated under reduced pressure to a relative density of 1.20 (60 ℃), add ethanol to make the alcohol content at 75%, let it stand, filter, and the filtrate reclaims ethanol under reduced pressure and concentrates to no alcohol smell; add water to dilute to contain salvianolic acid B15mg per 1ml, adjust the aqueous solution with 10% potassium hydroxide pH to 5.0, add 0.6% ZnCl 2 As a catalyst, heat conversion at 120°C for 3.5 hours, adjust the pH value of the conversion liquid to 2.5 with 15% hydrochloric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 5 mg of salvianolic acid A per 1 ml, pass through HPD-100 macroporous resin column layer Analysis and separation, the ratio of the loading amount of salvianolic acid A to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com