Application of salvianolic acid A freeze-dried powder injection for preparing medicines for preventing and/or treating cerebral thrombosis
A technology of freeze-dried powder injection and salvianolic acid, which is applied in the field of preparation of drugs for the prevention and/or treatment of cerebral thrombosis, and can solve problems such as the inability to guarantee the pharmacological effects of salvianolic acid A and the instability of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
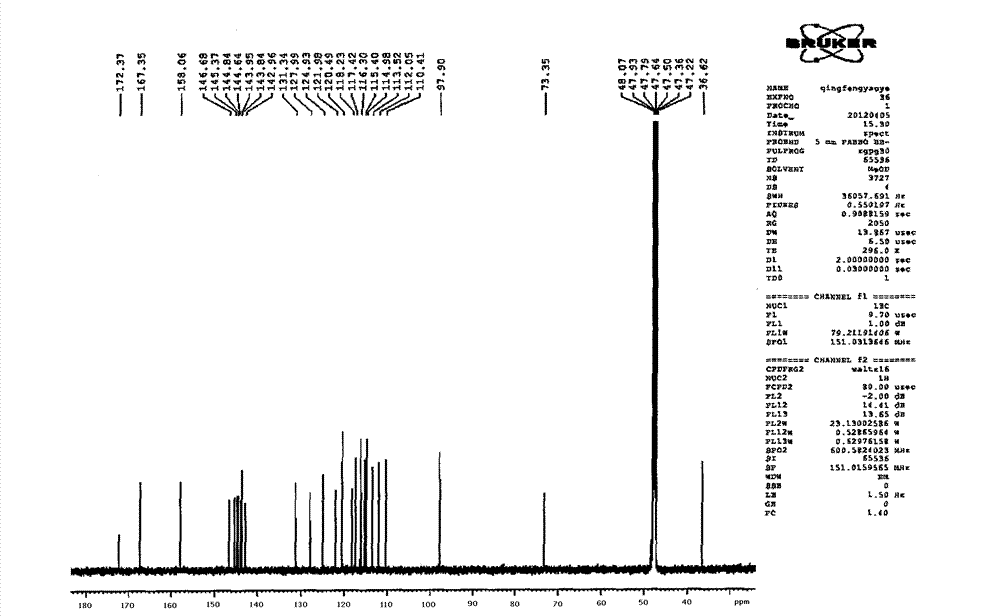
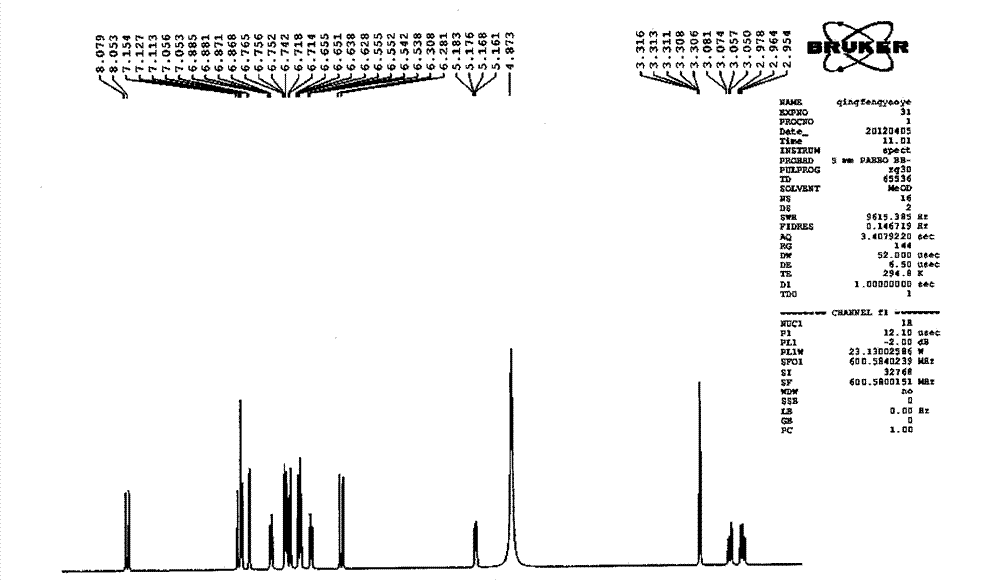
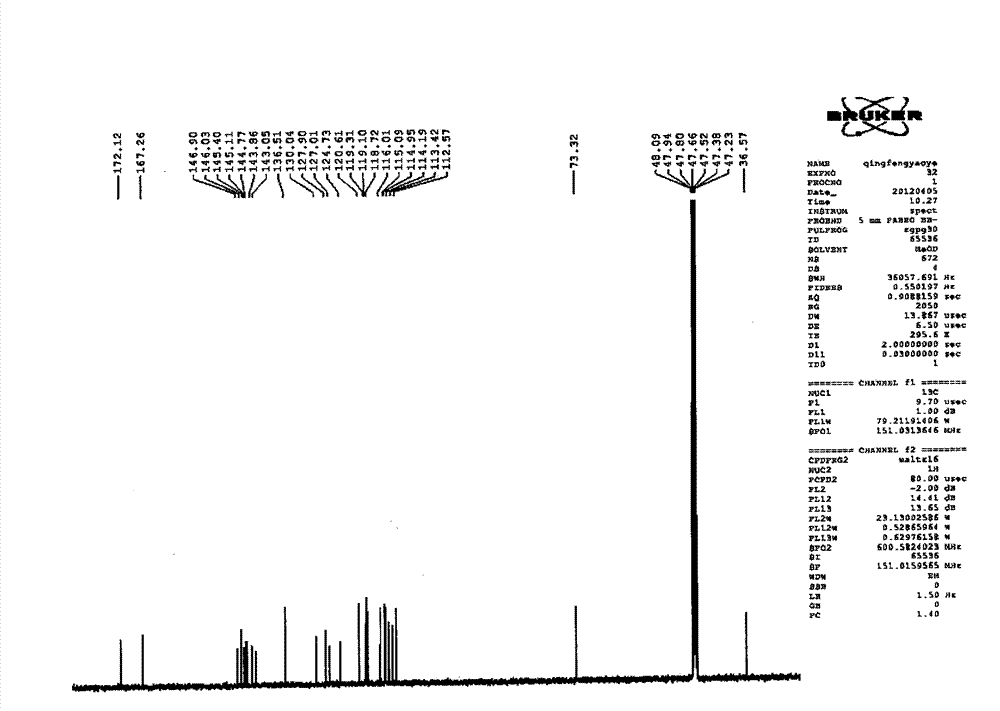
Image
Examples
Embodiment 1
[0107] Take Danshen medicinal material, crush it into 6-mesh granules, add 7 times the amount of 92°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, and warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to relative Density 1.20 (60°C), add ethanol to make the alcohol content at 70%, let it stand, filter, the filtrate is decompressed to recover ethanol and concentrate until it has no alcohol smell; add water to dilute to 20mg per 1ml of salvianolic acid B, use 10% for aqueous solution Adjust the pH to 4.0 with sodium hydroxide, add 0.5% ZnCl 2 As a catalyst, heat and transform at 120°C for 4 hours, adjust the pH value of the transformation liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and perform HPD-100 macroporous resin column chromatography For separation, the ratio of the loading amount of salv...
Embodiment 2
[0110] Take Salvia miltiorrhiza, crush it into particles with a diameter of about 2mm, add 7 times the amount of 90°C water each time, warm soak and extract 3 times, and stir at a speed of 25 rpm, and warm soak and extract for 3 hours each time; the extract is concentrated under reduced pressure to Relative density 1.18 (60°C), add ethanol to make the alcohol content 70%, let it stand, filter, the filtrate recovers ethanol under reduced pressure and concentrates to no alcohol smell; add water to dilute to contain salvianolic acid B20mg per 1ml, and use 10 mg of salvianolic acid B in the aqueous solution % NaOH to adjust the pH to 5.5, add 0.5% ZnCl 2 As a catalyst, heat and transform at 120°C for 4 hours, adjust the pH value of the transformation liquid to 2.5 with 20% phosphoric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 3 mg of salvianolic acid A per 1 ml, and perform HPD-100 macroporous resin column chromatography For separation, the rat...
Embodiment 3
[0113] Take Salvia miltiorrhiza, cut into decoction pieces, add 8 times the amount each time, soak in water at 85°C for 3 times, stir at a speed of 20 rpm, and extract with warm soaking for 2.5 hours each time; the extract is concentrated under reduced pressure to a relative density of 1.17 (60 ℃), add ethanol to make the alcohol content at 75%, let it stand, filter, the filtrate recovers ethanol under reduced pressure and concentrates to no alcohol smell; add water to dilute to contain salvianolic acid B15mg per 1ml, and adjust the aqueous solution with 10% hydroxide bell pH to 5.0, add 0.6% ZnCl 2 As a catalyst, heat conversion at 120°C for 3.5 hours, adjust the pH value of the conversion liquid to 2.5 with 15% hydrochloric acid, centrifuge, concentrate the supernatant under reduced pressure to contain 5 mg of salvianolic acid A per 1 ml, pass through HPD-100 macroporous resin column layer Analysis and separation, the ratio of the loading amount of salvianolic acid A to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com