Application of salvianolic acid C in preparation of drugs for prevention and treatment of hyperuricemia
A technology of hyperuricemia and salvianolic acid, which is applied in the field of medicine and can solve the problems that have not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation and structure identification of salvianolic acid C:
[0016] Take 10 kg of Danshen medicinal material, decoct it with water, filter the extract, concentrate under reduced pressure to obtain extract, dissolve the extract in water, adjust the pH value to be acidic, and use macroporous adsorption resin to purify to obtain total phenolic acid and total phenolic acid. The acid was purified by Sephadex LH-20 column and ODS column to obtain compound salvianolic acid C.
[0017] According to HRESI-MS, the molecular formula is C 26 h 20 o 10 ([M-H] - m / z 491.0994, calculated: 491.0984). 1 H-NMR (300MHz, CDCl 3 )δppm: 6.73 (1H, d, J=8.25Hz, H-5), 7.36 (1H, d, J=8.25Hz, H-6), 7.92 (1H, d, J=16.0Hz, H-7) , 6.46(1H, d, J=16.0Hz, H-8), 6.72(1H, d, J=8.0Hz, H-5″), 6.66(1H, d, J=8.0Hz, 1.9Hz, H- 6″), 6.80 (1H, d, J=1.9Hz, H-2″), 3.14 (1H, dd, J=14.4Hz, 4.3Hz, H-7α″’), 3.06 (1H, dd, J= 14.4Hz, 8.2Hz, H-7β″’), 5.25 (1H, dd, J=8.2Hz, 4.3Hz, H-8″), 7.40 (1H, d, J=2.0Hz,...
Embodiment 2
[0020] Xanthine oxidase inhibitory activity of salvianolic acid C
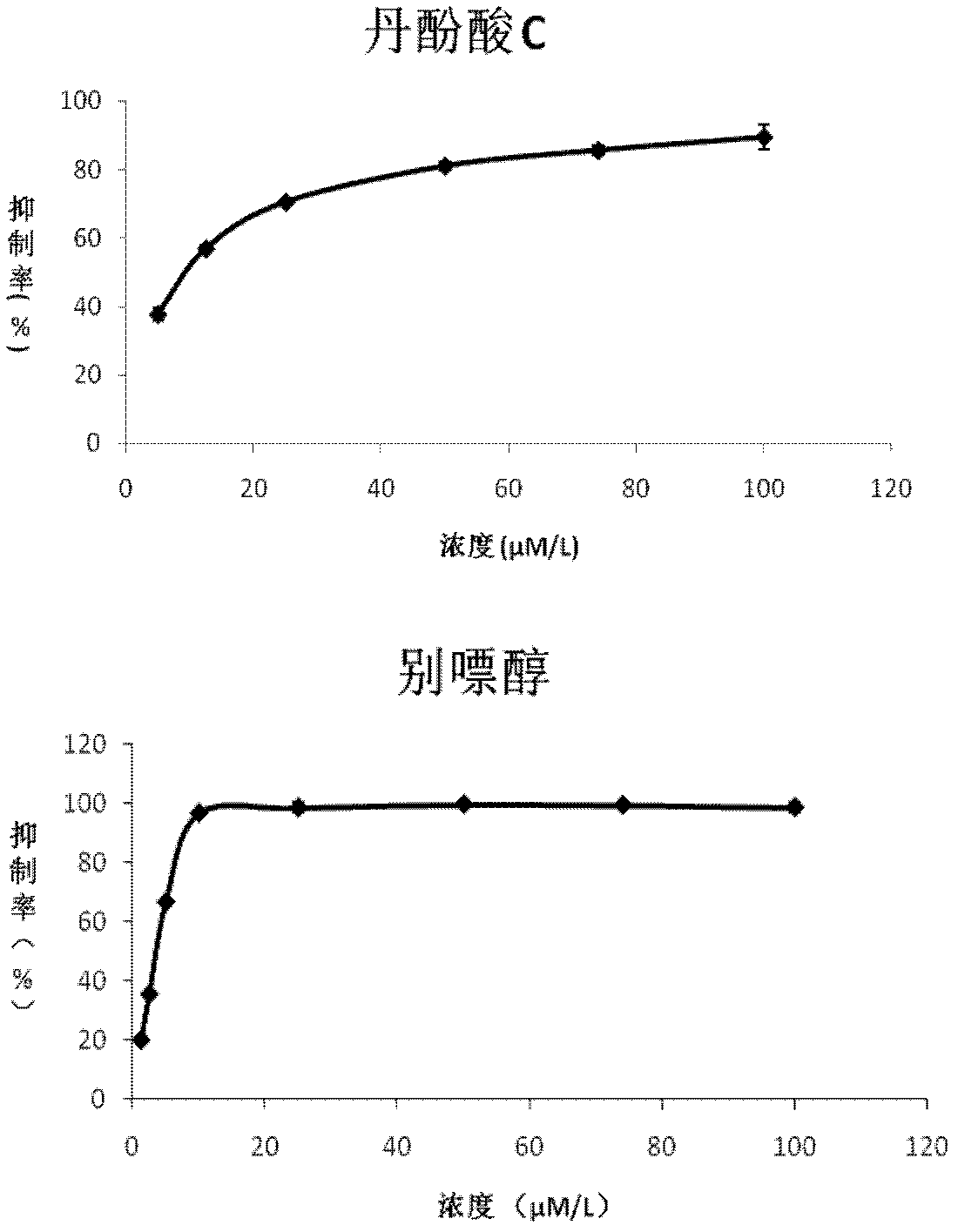
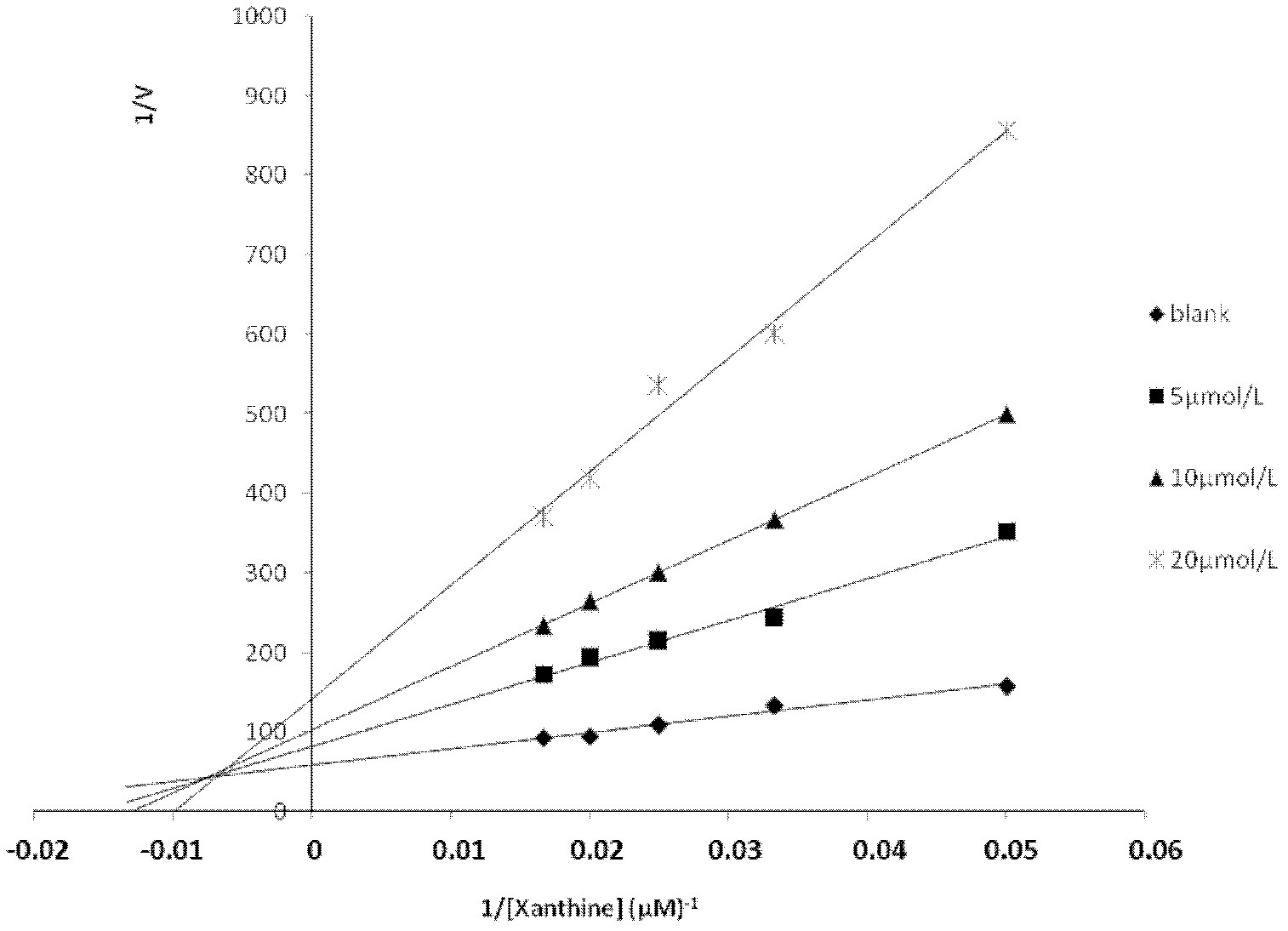
[0021] The xanthine oxidase inhibitory activity of salvianolic acid C was determined by the uric acid production method. The specific experimental method is as follows: fix the concentration of xanthine oxidase (Sigma company, batch number 119K3793) to 0.2 units, add different concentrations of salvianolic acid C (5 μmol / L, 12.5 μmol / L, 25 μmol / L, 50 μmol / L, 75 μmol / L, 100 μmol / L), after incubating at 25°C for 3 minutes, add xanthine 120 μmol / L, measure the UV absorbance at 295 nm, and calculate the inhibition rate. The result is as figure 1 , whose IC 50 =9.07μmol / L, the IC of the positive control drug allopurinol 50 =2.55μmol / L, indicating that salvianolic acid C has strong xanthine oxidase inhibitory activity. Calculate the kinetic constant of salvianolic acid C inhibiting xanthine oxidase according to Lineweaver-Burk's plotting method. The concentration of xanthine oxidase was 0.24units, and the reac...
Embodiment 3
[0023]Direct interaction between salvianolic acid C and xanthine oxidase
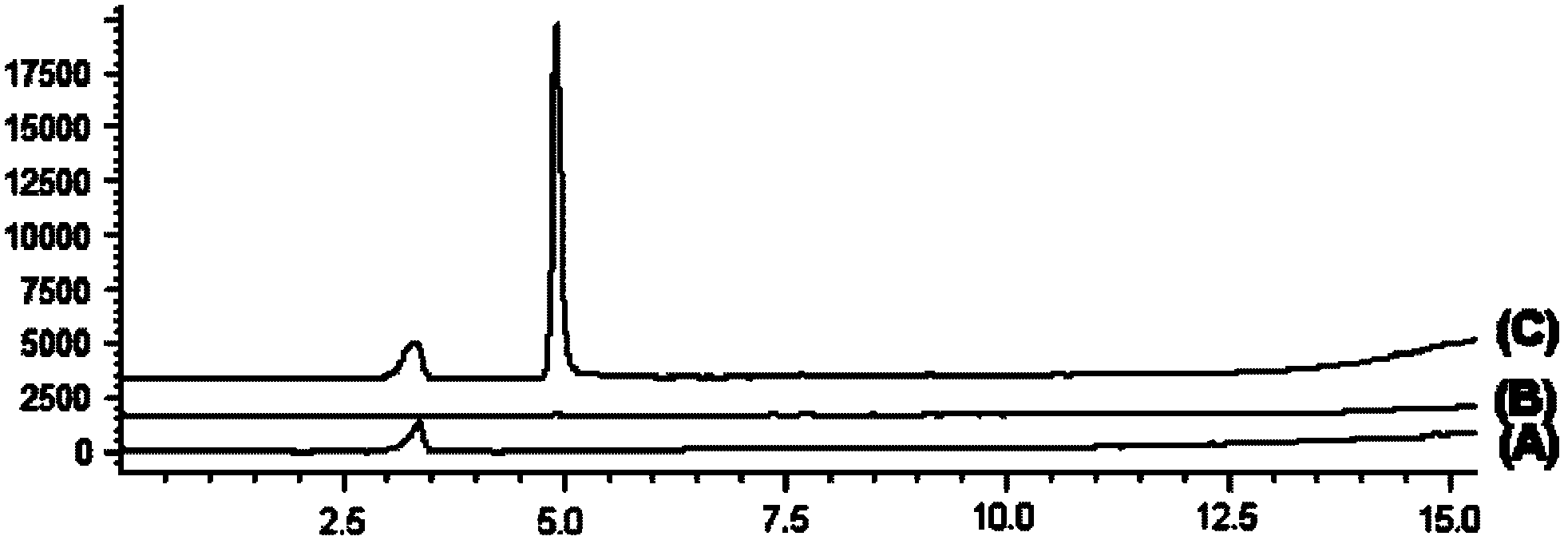
[0024] According to the target protein affinity-two-dimensional turbulent flow chromatography-LC / MS online analysis method [Jian-Liang Zhou, Jing-Jing An, Ping Li, Hui-Jun Li, Yan Jiang, Jie-Fei Cheng.Two-dimensional turbulent flow Chromatography coupled on-line to liquid chromatography-mass spectrometry for solution-based ligand screening against multiple proteins.Journal of Chromatography A, 2009, 1216: 2394-2403], the identification of salvianolic acid C and xanthine oxidase (XO) can be directly interaction. Salvianolic acid C with a concentration of 20 μmol / L and xanthine oxidase were incubated at 25°C in the dark for 1 hour, and 20 μL of sample was injected for two-dimensional eddy current chromatography-MSD analysis and detection. At the same time, 20 μL of phosphate buffer (PB) and 20 μmol / L of Salvianolic acid C was used as a control. The result is as image 3 , showing that only salvianolic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com