Chan tablet liquid chromatography-mass spectrometry determination method
A liquid mass spectrometry and determination method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of undisclosed content determination methods and undetectable components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
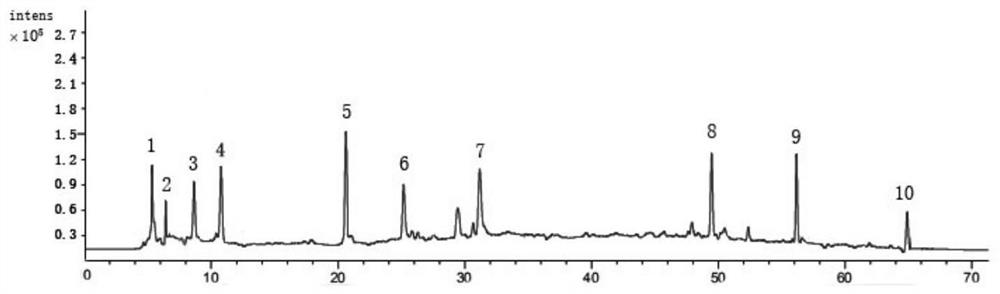
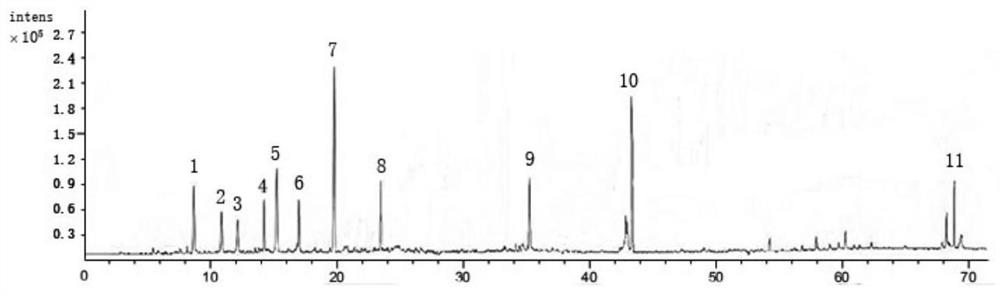
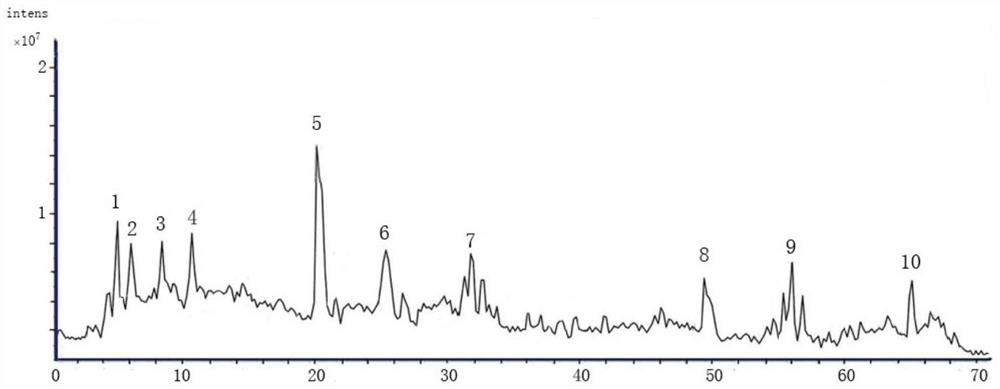
[0085] Preparation of reference solution
[0086] Take psoralen, different psoralen, hyperoside, quercetin 3-O-glucuronide, isoquercetin, sennoside B, astragalus glycoside, sennoside A, Arnicic acid, shikonian acid, salvianolic acid B, nuciferine, salvianolic acid A, psoralen, isopsoralen, salvianolic acid C, rhein, neopsoralen isoflavones, psoralen Appropriate amounts of reference substances of Ning, cryptotanshinone, and bakuchiol were accurately weighed, placed in 10mL volumetric flasks, dissolved in methanol to constant volume, and prepared into concentrations of 227.6 μg / mL, 128.3 μg / mL, and 169.5 μg / mL, respectively. mL, 248.4μg / mL, 135.7μg / mL, 185.3μg / mL, 119.2μg / mL, 84.2μg / mL, 193.8μg / mL, 74.8μg / mL, 236.1μg / mL, 149.3μg / mL, 106.2μg / mL mL, 196.4μg / mL, 125.8μg / mL, 62.7μg / mL, 174.8μg / mL, 58.3μg / mL, 44.2μg / mL, 138.3μg / mL, 96.4μg / mL stock solutions of reference substances.
[0087] Take psoralen, isopsoralen, hyperoside, isoquercitrin, nuciferine, psoralen, isopsoralen, ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com