Preparation method and quality control method of traditional Chinese medicine Danmo capsule
A technology of traditional Chinese medicine and Danmo, applied in the field of preparation and quality control of traditional Chinese medicine Danmo capsules, can solve problems such as difficulty in swallowing, large volume, and no production process optimization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
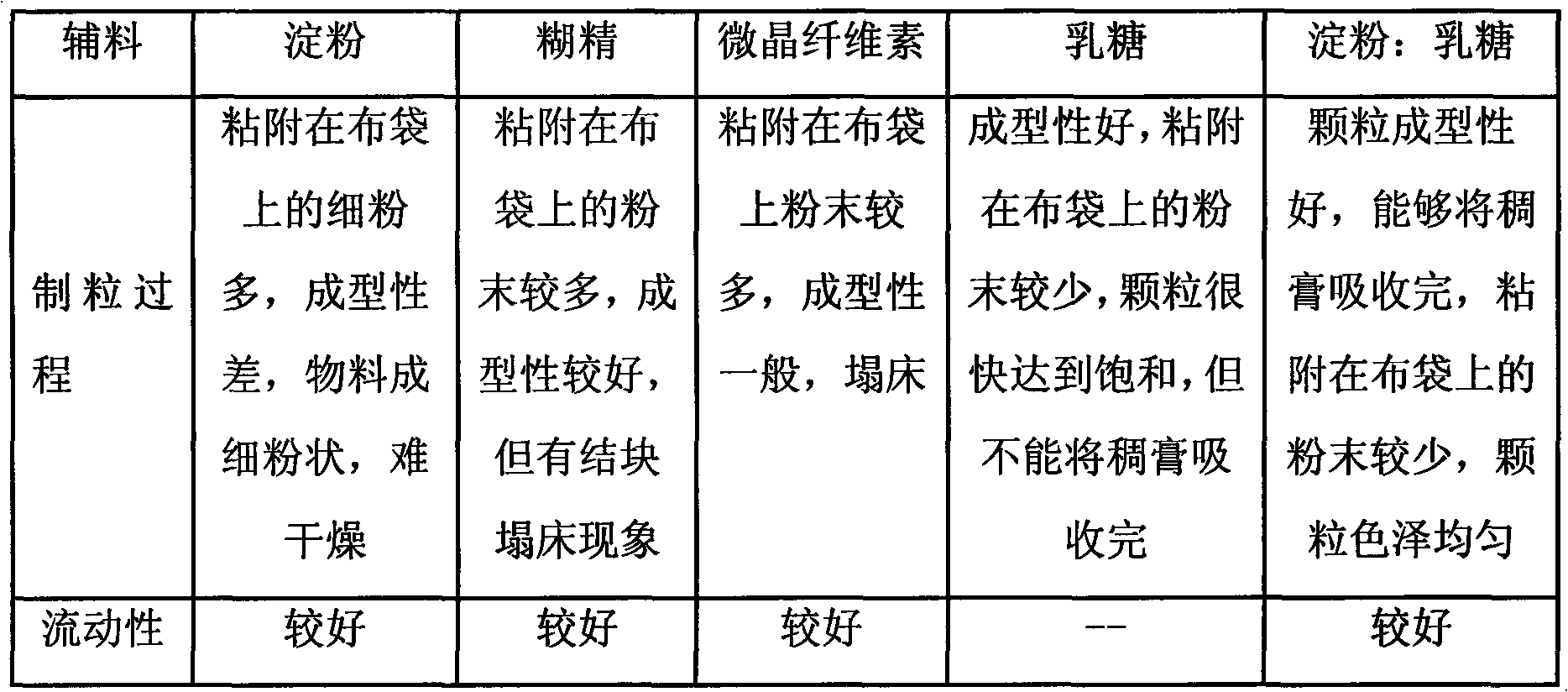
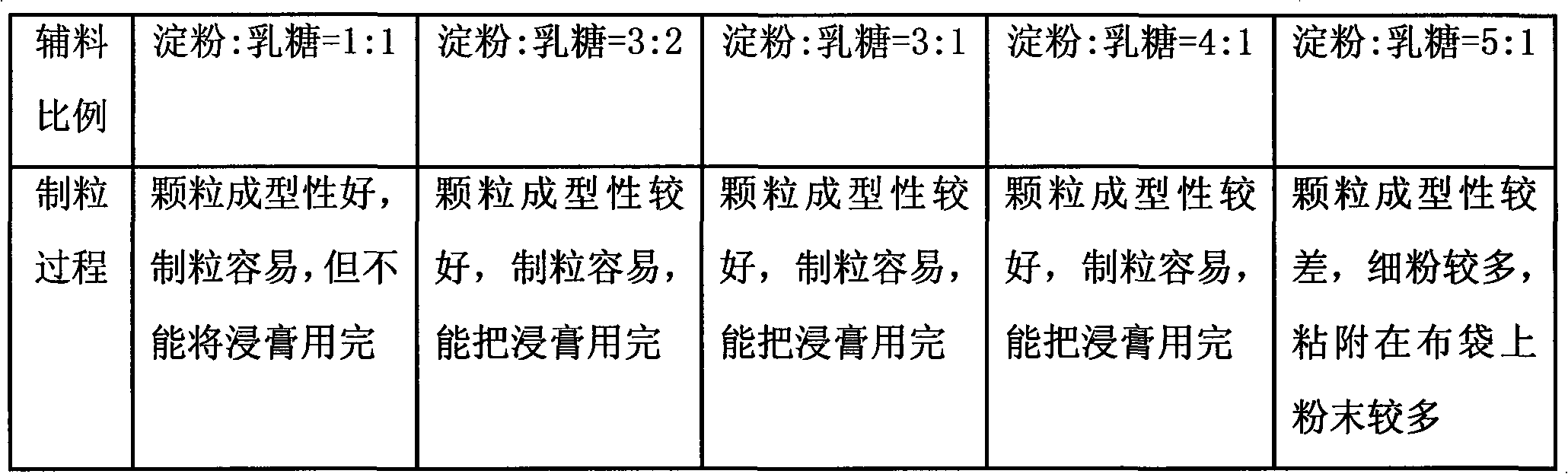
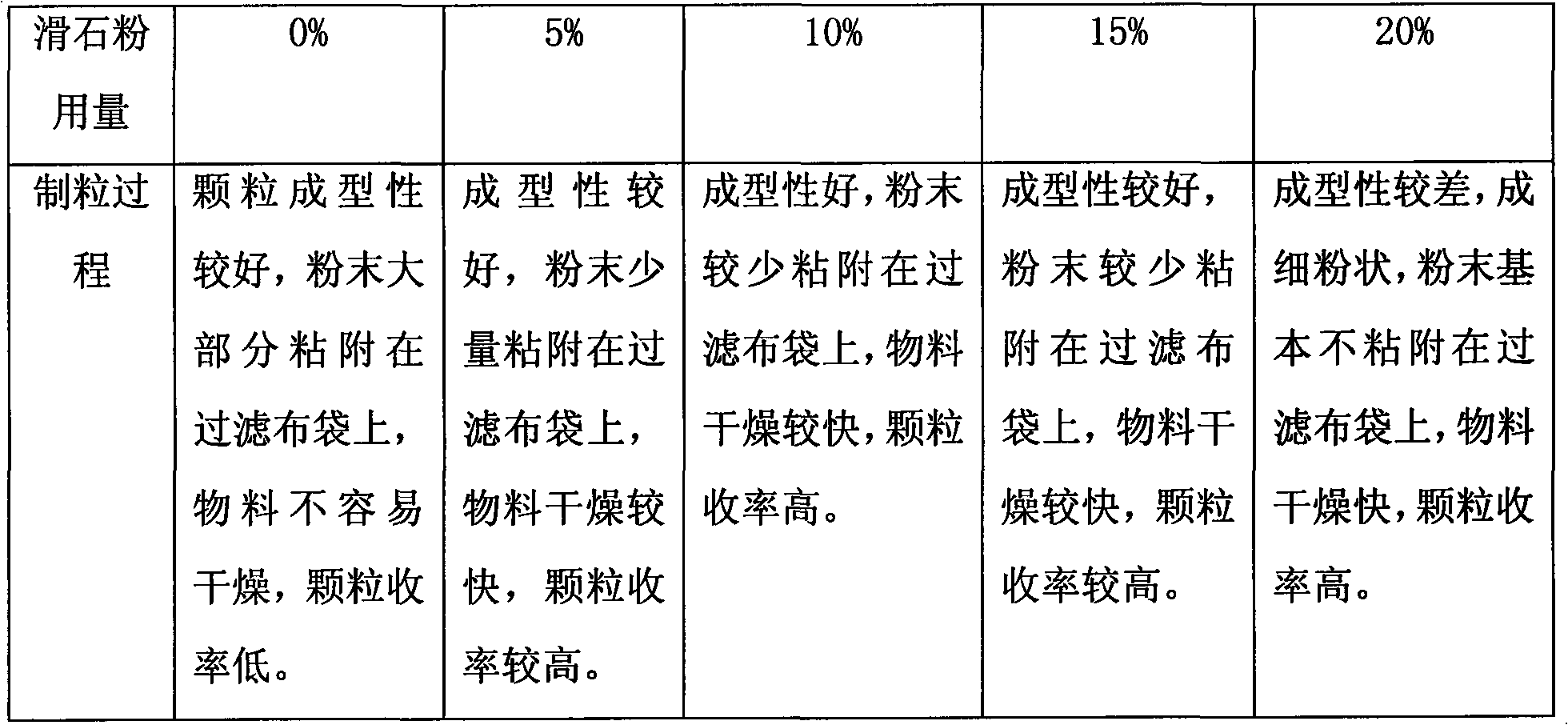
[0019] Take Eclipta, Ligustrum lucidum, Danshen, honeysuckle, motherwort, rehmannia glutinosa, madder, Digupi and Scorpion nine herbs, add 12 times the water, decoct once for 2.5 hours in total, combine the decoction, filter, Concentrate the filtrate to a relative density of 1.05-1.10g / mL (50°C) clear paste, add ethanol to make the alcohol content reach 60%, stir while adding, let stand for 15 hours, take the supernatant to recover ethanol, concentrate to a relative density of 1.15-1.20g / mL (50°C) thick paste for later use, add auxiliary materials (starch, lactose and talcum powder, wherein the dosage of starch and lactose is 3:2, and the dosage of talc powder is 5%) in a one-step granulator to preheat Finally, keep the air inlet temperature at 80°C, use the above-mentioned thick paste as a binder, spray into the thick paste for one-step granulation, granulate after drying, add additional auxiliary materials (0.5% magnesium stearate and 0.5% talcum powder), and mix well , fill...
Embodiment 2
[0021] Take Eclipta, Ligustrum lucidum, Danshen, honeysuckle, motherwort, rehmannia glutinosa, madder, Digupi and Scorpion nine herbs, add 8 times the water, decoct 3 times, 1.5 hours each time, combine the decoction, filter , the filtrate is concentrated to a relative density of 1.15 ~ 1.20g / mL (50 ℃) clear paste, add ethanol to make the alcohol content reach 80%, stir while adding, let stand for 30 hours, take the supernatant to recover ethanol, concentrate to the relative density To make a thick paste of 1.20-1.25g / mL (50°C) for later use, add auxiliary materials (starch, lactose and talcum powder, wherein the dosage of starch and lactose is 4:1, and the dosage of talcum powder is 15%) in a one-step granulator. After heating, keep the air inlet temperature at 90°C, use the above-mentioned thick paste as a binder, spray into the thick paste for one-step granulation, granulate after drying, add additional auxiliary materials (0.5% magnesium stearate and 0.5% talcum powder), an...
Embodiment 3
[0023]Take Eclipta, Ligustrum lucidum, Salvia, Honeysuckle, Motherwort, Rehmannia glutinosa, Rubia, Digupi and Scorpion nine herbs, add 10 times of water, decoct twice, each time for 2 hours, combine the decoction, filter , the filtrate is concentrated to a relative density of 1.10 ~ 1.15g / mL (50 ℃) clear paste, add ethanol to make the alcohol content reach 70%, stir while adding, let stand for 24 hours, take the supernatant to recover ethanol, concentrate to the relative density To make a thick paste of 1.25-1.30g / mL (50°C) for later use, add auxiliary materials (starch, lactose and talcum powder, wherein the dosage of starch and lactose is 3:1, and the dosage of talcum powder is 10%) in a one-step granulator. After heating, keep the air inlet temperature at 80°C, use the above-mentioned thick paste as a binder, spray into the thick paste for one-step granulation, granulate after drying, add additional auxiliary materials (0.5% magnesium stearate and 0.5% talcum powder), and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com