Pharmaceutical composition for treating myocardial infarction
A technology of acute myocardial infarction and composition, applied in the field of pharmaceutical composition for the treatment of myocardial infarction, can solve the problems of increased mortality rate of model rats, reduction of infarct size of model rats of acute myocardial infarction, etc., and achieve reduction of toxic and side effects in vivo and in vitro , reduce the size of myocardial infarction, and enhance the effect of myocardial protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
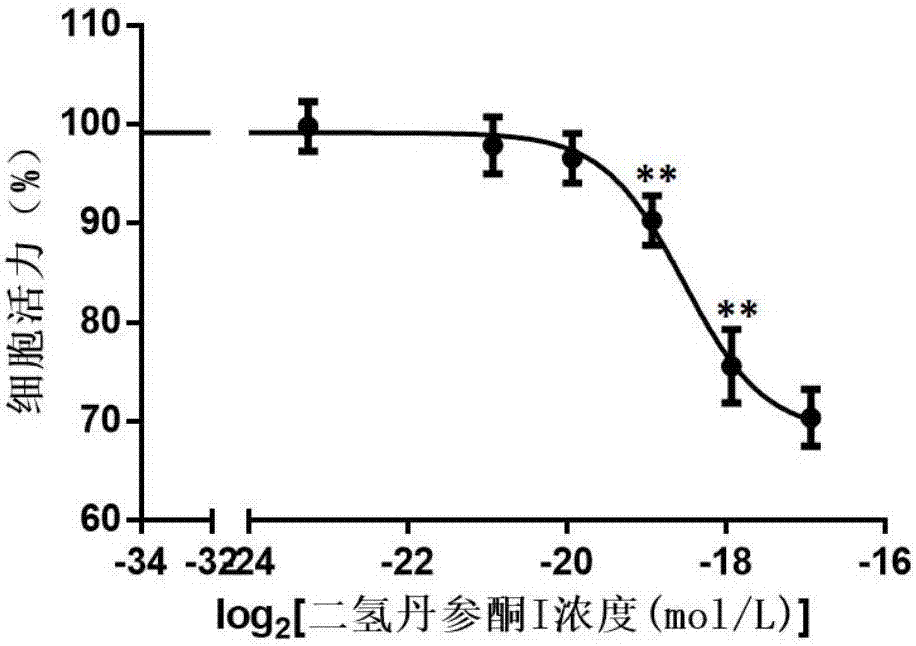
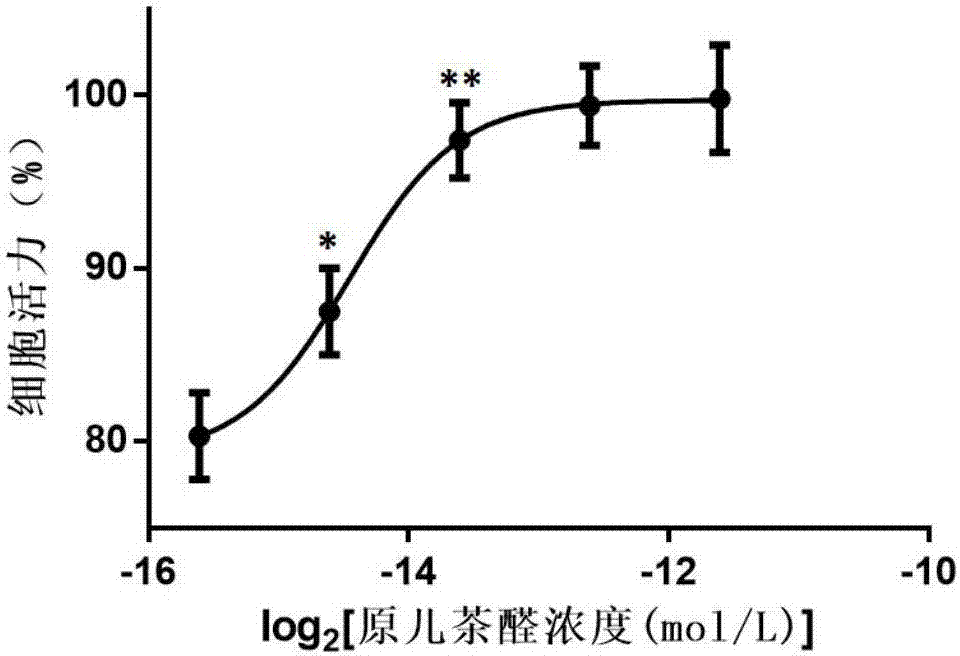
[0059] Embodiment 1: the attenuation effect of protocatechualdehyde to dihydrotanshinone I
[0060] 1. Experimental materials
[0061] 1.1 Instruments and equipment
[0062] Ultra-clean bench (Thermo Scientific 1300A2, USA); multifunctional microplate reader FLUOstar Omega (BMG LABTECH, Germany); electronic scale (Tianjin Tianma Hengji Instrument Co., Ltd.); Sartorius analytical balance (Beijing Doris Balance Co., Ltd.); BL -420S biological function experiment system (Chengdu Taimeng Technology Co., Ltd.); HX-100E small animal ventilator (Chengdu Taimeng Technology Co., Ltd.); HW-1000 water bath (Chengdu Taimeng Technology Co., Ltd.).
[0063] 1.1 Reagents
[0064] Standard products: dihydrotanshinone I and protocatechualdehyde were purchased from Chengdu Mansite Biotechnology Co., Ltd.
[0065] Reagents: DMEM high glucose medium, fetal bovine serum, 100× penicillin and streptomycin (Thermo Fisher, USA); type II collagenase (Worthington, USA); 5-bromodeoxyuridine (sigma, ...
Embodiment 2
[0127] Embodiment 2: the synergistic effect of protocatechualdehyde and dihydrotanshinone I
[0128] 1.1 Instruments and equipment
[0129] Multifunctional microplate reader FLUOstar Omega (BMG LABTECH, Germany); 80-2 desktop low-speed centrifuge (Shanghai Medical Instrument (Group) Co., Ltd. Surgical Instrument Factory); 5804R desktop refrigerated high-speed centrifuge (Eppendorf, Germany); Sartorius analysis Balance (Beijing Doris Balance Co., Ltd.) electronic scale (Tianjin Tianma Hengji Instrument Co., Ltd.); Sartorius analytical balance (Beijing Doris Balance Co., Ltd.); BL-420S biological function experiment system (Chengdu Taimeng Technology Co., Ltd.); HX- 100E small animal ventilator (Chengdu Taimeng Technology Co., Ltd.); HW-1000 water bath (Chengdu Taimeng Technology Co., Ltd.).
[0130] 1.2 Reagents
[0131] Standard products: dihydrotanshinone I, protocatechualdehyde, all purchased from Chengdu Mansite.
[0132] Reagents: urethane (Sinopharm Chemical Reagent Co...
Embodiment 3
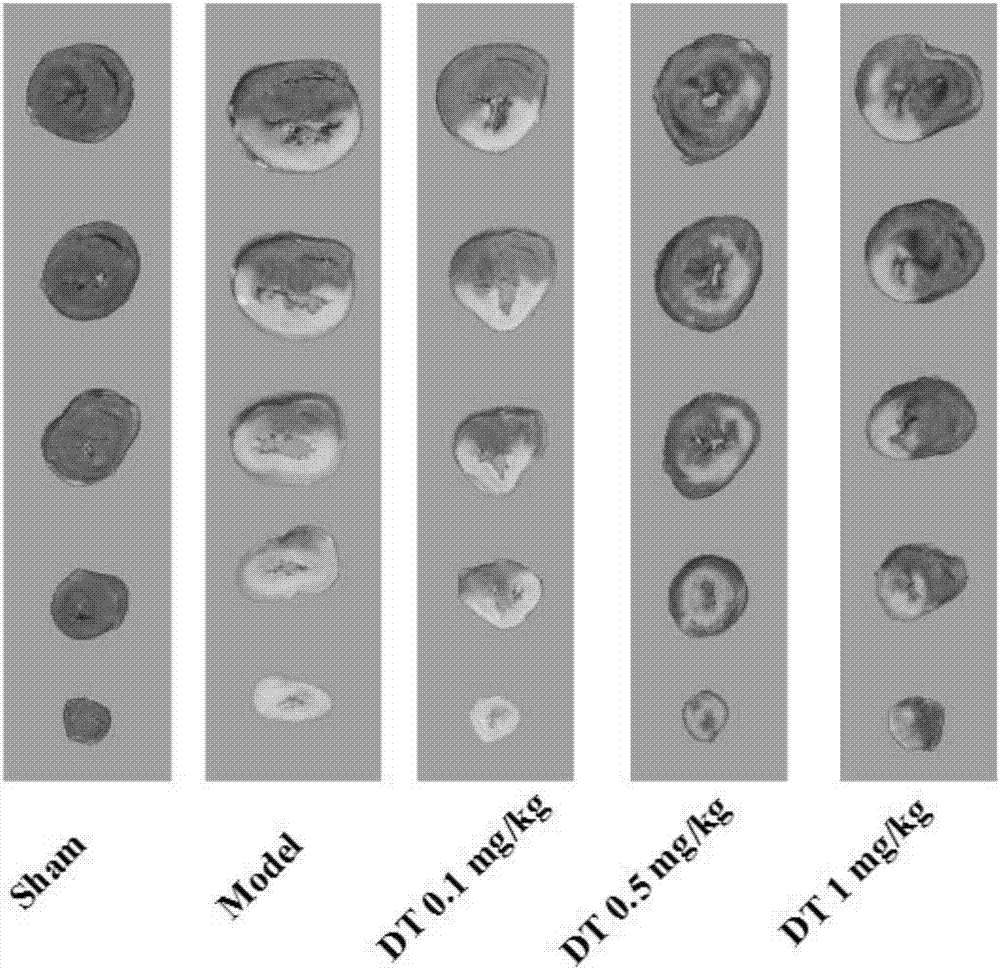
[0156] Embodiment 3: Danshensu (DSS) to the attenuation and synergistic effect of Tanshinone I (TI)
[0157] The experimental materials and experimental methods are the same as those in Example 1 and Example 2, and the experimental results are as follows.
[0158] 1. In vitro study on the cytotoxicity of tanshinone I and the attenuation of tanshenin
[0159] The primary cardiomyocytes of suckling rats were treated with tanshinone I for 24 hours, and the cell viability was measured after 1 week of culture. The experimental results showed that when the concentration of tanshinone I was higher than 2 μM, there was a certain cytotoxic effect (compared with the control group, P Figure 10 and Table 6. When the administration concentration of tanshinone I was 4 μM, different concentrations of danshensu were given. When the concentration of danshinone was higher than 37.5 μM, the cell death caused by tanshinone I could be effectively reversed (P Figure 11 and Table 7.
[0160] Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com