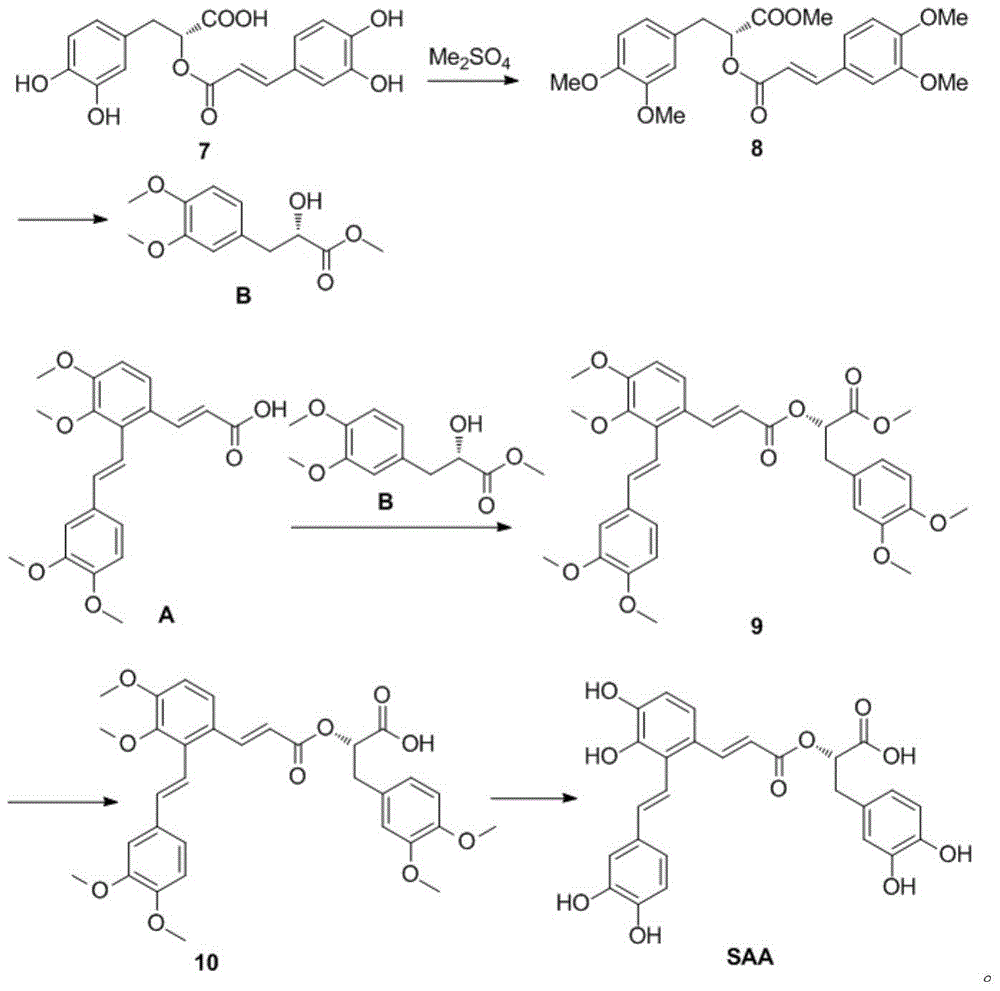
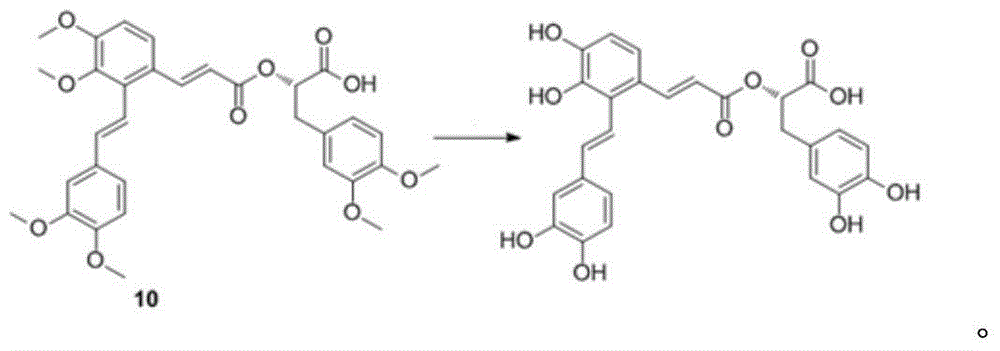
Synthetic method for salvianolic acid A
A synthesis method and technology of salvianolic acid, applied in the field of synthesis of salvianolic acid A, can solve problems such as obtaining salvianolic acid A and difficult natural products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
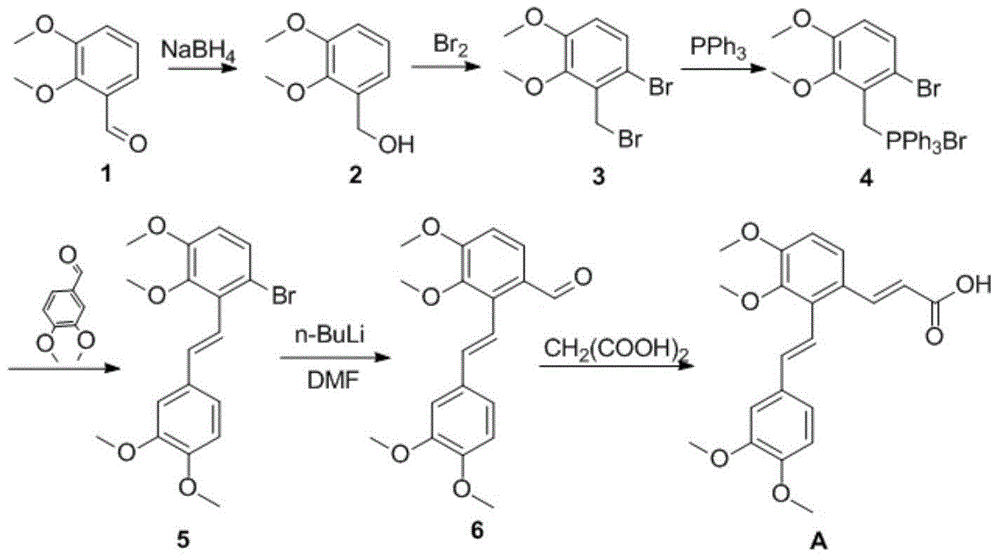
[0027] The synthesis of embodiment 1 compound 2
[0028]
[0029] Dissolve 34.0 g (0.21 mol) of 2,3-dimethoxybenzaldehyde (1) in 120 ml of methanol and keep stirring. Weigh 4.7g (0.124mol) of sodium borohydride and carefully add to the reaction solution in batches. Then, it was stirred at room temperature for 1 h, and analyzed by TLC, the reaction was complete. The reaction was stopped, and the solvent methanol was removed by rotary evaporation. The residue was dissolved in dichloromethane, washed with water, washed with saturated brine, and dried over anhydrous magnesium sulfate. Filter and remove the solvent to obtain a light gray viscous liquid, which becomes a solid after standing and weighs 34.0 g (yield 99%). spare.
Embodiment 2
[0030] The synthesis of embodiment 2 compound 3
[0031]
[0032]Weigh 16.8g (0.1mol) of 2,3-dimethoxybenzyl alcohol (2) and dissolve it in 450ml of dichloromethane to completely dissolve it, and cool down to 0°C in an ice bath. 17.6g (5.64ml) of liquid bromine was dissolved in 50ml of dichloromethane and placed in a constant pressure dropping funnel. During the dropwise addition, the temperature was kept below 5°C. After about 0.5 hours, the dropwise addition was completed, and the ice bath was removed, and the temperature rose to room temperature. The reaction was monitored by TLC over time. After about 2 hours, the reaction was completed. Immediately, 100ml of 1M sodium bicarbonate solution was added to the solution, extracted, washed with water, washed with saturated brine, and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation to obtain a yellow viscous substance, which was recrystallized from methanol to obtain a yellow crystal, wh...
Embodiment 3
[0033] The synthesis of embodiment 3 compound 4
[0034]
[0035] Dissolve 22g of compound 3 in 500ml of toluene, add 18.6g of triphenylphosphine, and stir vigorously. And began to heat up to reflux, after stirring for about 10 minutes, the solution began to become cloudy, and a white solid was produced. After reflux for 4 hours, it was cooled to room temperature, and a large amount of white precipitate was formed, which was filtered and dried in vacuo. 38.5 g of white solid were obtained, yield 95%. Mp169°C. 1H-NMR (400MHz, CDCl3): 3.75(s, 3H), 3.79(s, 3H), 5.16(d, 2H, J=14Hz), 6.75(d, 1H, J=8.8Hz), 7.05(d, 1H, J=8.8Hz), 7.58-7.82 (m, 15H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com