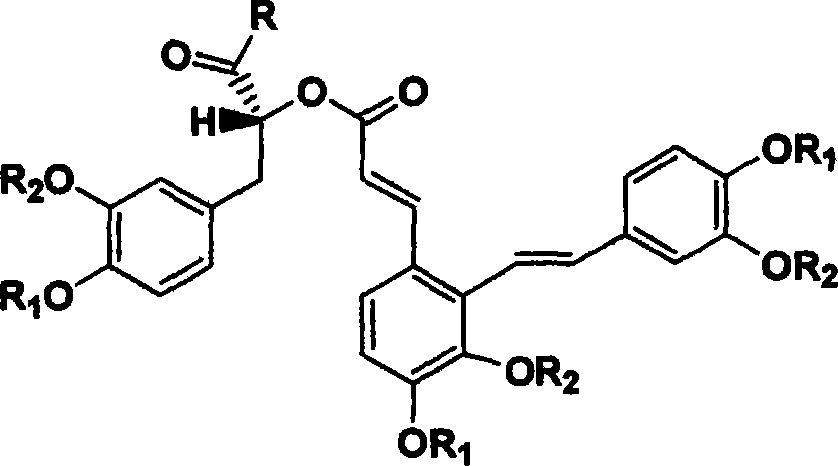
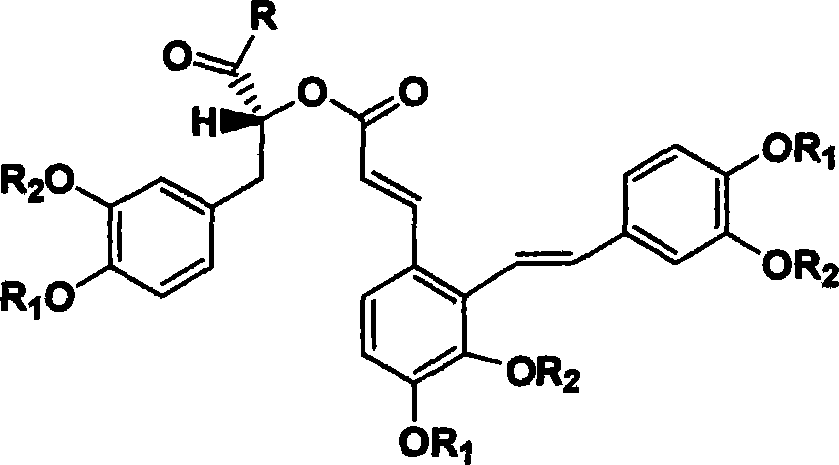
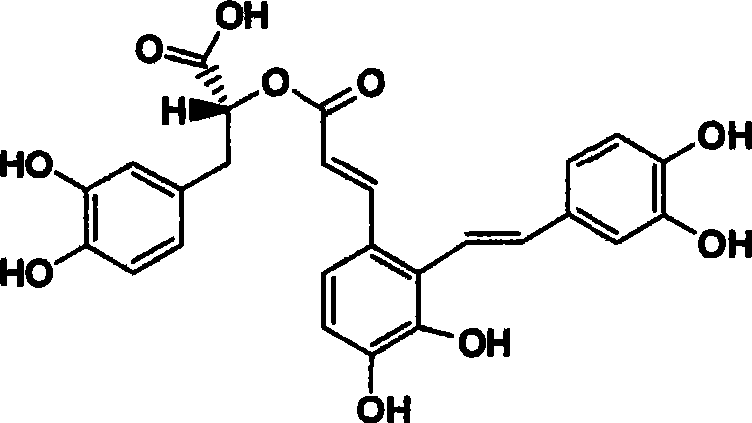
Derivant of salviol acidn A and its application as antioxidant
A technology of salvianolic acid and antioxidant, which is applied in the application field of derivatives as antioxidants, can solve problems such as poor absorption, insufficient purity, and differences in active bulk compounds, and achieve good oral absorption, no drug side effects, and reliable quality control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of salvianolic acid A
[0033] 150Kg Salvia miltiorrhiza was crushed (40-80 mesh), extracted four times with deionized water (600L×4 times, 80-100°C, 2 hours), combined the extracts, filtered, put on HPD100A resin column (Ф500×1500), first Wash with 1500L water, then elute with 50% methanol, concentrate the 50% methanol eluate under reduced pressure at 45-60°C to a dry paste, dissolve the concentrate in 100L ethanol, let stand at 4°C for 24 hours, and filter. The filtrate was concentrated under reduced pressure at 45-60°C to a dry paste, and then the concentrated solution was dissolved in 100L water, extracted twice with chloroform (100L×2 times), extracted three times with ethyl acetate (100L×3 times), and the chloroform layer Chloroform was recovered under reduced pressure and then discarded. The concentrated solution was extracted with ethyl acetate and the aqueous phase was discarded. The ethyl acetate layers were combined, dehydrated ...
Embodiment 2
[0045] Embodiment 2: the preparation of salvianolic acid A ethyl ester
[0046] 494g salvianolic acid A was dissolved in 10L absolute ethanol (containing 0.1mol H 2 SO 4 ), add 2L of chloroform, reflux with a device equipped with a fractionation column and a water separator until no more water comes out, add 20L of water to dilute, and then put it on a Sephadex LH20 column (Ф150×1500), sequentially use 100L of 50% methanol solution, methanol The solution was eluted, and the methanol eluate was concentrated under reduced pressure at 45-60° C. to obtain 480 g of light yellow solid (92% yield).
[0047] Its molecular structure is:
[0048]
Embodiment 3
[0049] Embodiment 3: the preparation of salvianolic acid A methyl ester
[0050] Dissolve 494g of salvianolic acid A in 5L of methanol, then carefully add 200ml of acetyl chloride dropwise, let it stand overnight, add 10L of water to dilute, put it on a Sephadex LH20 column (Ф150×1500), and successively elute with 100L of 50% methanol solution and methanol solution , the methanol eluate was concentrated under reduced pressure at 45-60° C. to obtain 482 g of light yellow solid (95% yield).
[0051] Its molecular structure is:
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com