Medicament composition
A composition and drug technology, applied in the field of medicine, can solve the problems of no effective ingredient research, no research on the formulation of salvianolic acid A prescription, no research on prescription formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment approach 1
[0101] Scheme 1: 0.2 parts by weight of salvianolic acid A;
[0102] Scheme 2: 0.4 parts by weight of salvianolic acid A;
[0103] Scheme 3: 0.6 parts by weight of salvianolic acid A;
[0104] Scheme 4: 0.8 parts by weight of salvianolic acid A;
[0105] Scheme 5: 1 part by weight of salvianolic acid A;
[0106] Scheme 6: 2 parts by weight of salvianolic acid A;
[0107]Experimental method: Experimental method: take healthy SD rats, weighing 240-260g, and randomly divide them into blank control group and experimental scheme group. Placed in the same environment for pre-feeding for 2 days, free to eat and drink. After the pre-breeding, carry out the experiment, weigh the animals, inject 20% urethane at 0.6ml / 100g intraperitoneally, after the anesthesia is satisfactory, fix it on the mouse board in a supine position, intubate the trachea, connect the ventilator, and use a tidal volume of 10-12ml , The frequency of 70 times / min is given to exhale, continuous positive press...
experiment approach 2
[0111] Scheme 1: 1 part by weight of American ginsenoside;
[0112] Scheme 2: 5 parts by weight of American ginseng saponins;
[0113] Scheme 3: 10 parts by weight of American ginseng saponins;
[0114] Scheme 4: 20 parts by weight of American ginseng saponins;
[0115] Scheme 5: 30 parts by weight of American ginseng saponins;
[0116] Scheme 6: 40 parts by weight of American ginseng saponins;
[0117] Scheme 7: 50 parts by weight of American ginseng saponins;
[0118] Experimental method: Carry out according to the above-mentioned experimental method, and the experimental results are shown in Table 9:
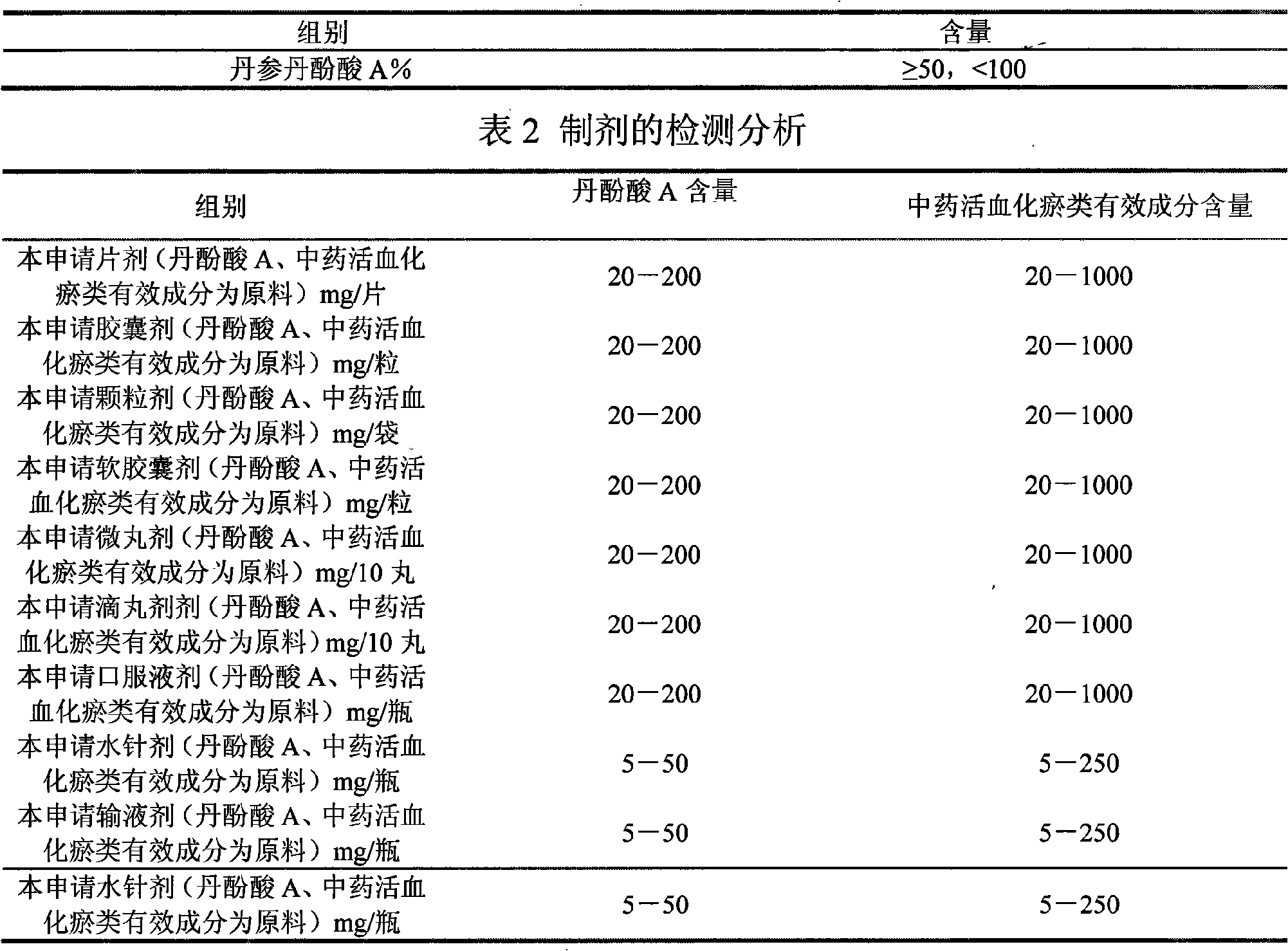
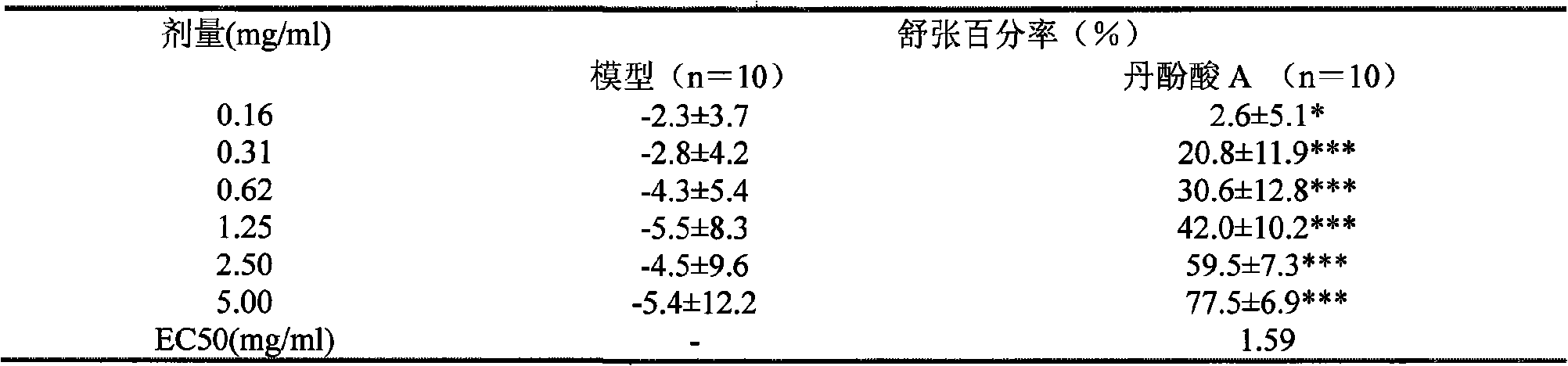
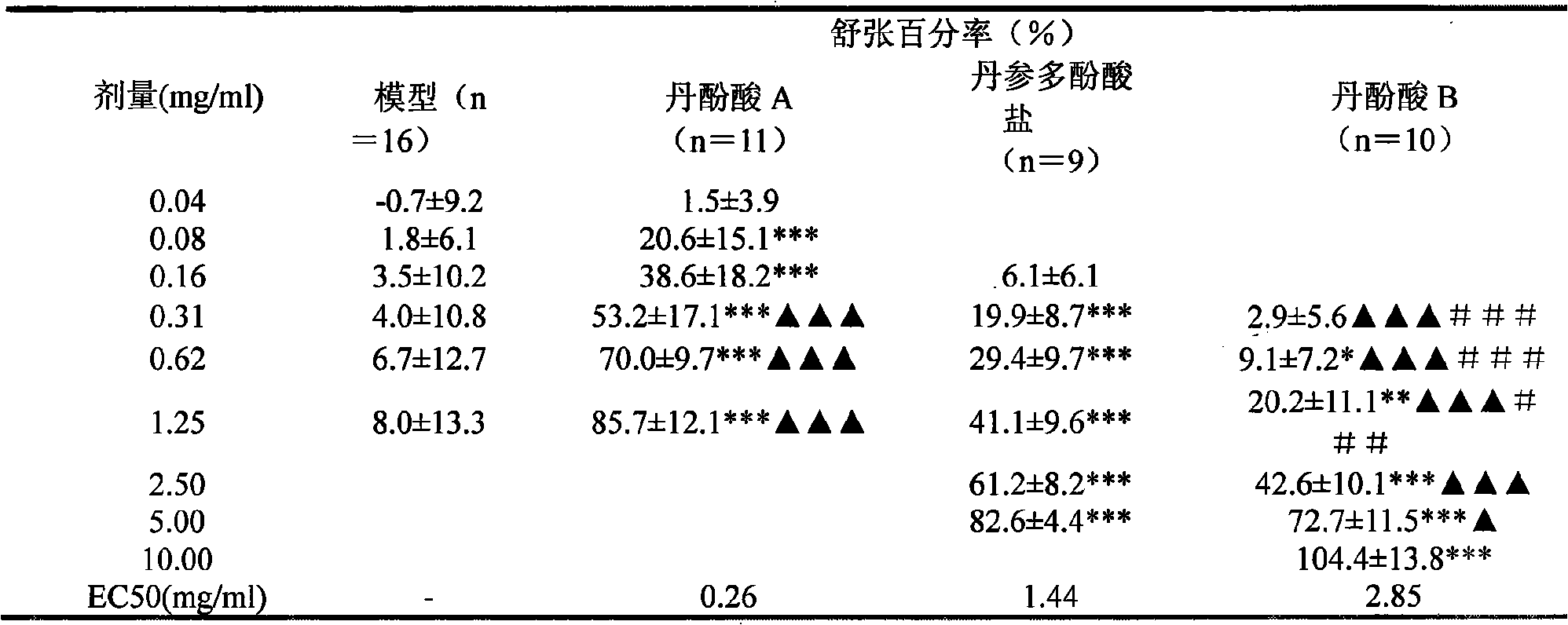
[0119] Table 8 Experimental results of different schemes of salvianolic acid A
[0120]
[0121] Table 9 Experimental results of different schemes of American ginseng saponins
[0122]
[0123] Note: The above experimental scheme was administered by intragastric administration, and the experimental results were similar to the above experimental results.
[0124] ...
Embodiment 1
[0290] Preparation of salvianolic acid A:
[0291] Extract the salvia miltiorrhiza with 70% ethanol solution to obtain the alcohol extract, concentrate the ethanol to the utmost, adjust the pH value to 4.5, 120°C temperature, and a gauge pressure of 0.10MPa, and heat for 4 hours; the solution is filtered, and the filtrate is passed through HPD-450 Pore resin column chromatography separation, first eluted with water and 20% dilute ethanol to remove impurities, then eluted with 50% ethanol, collected the eluate, concentrated, and dried to obtain salvianolic acid A with a content of 53.8% .
[0292] or
[0293]Extract the salvia miltiorrhiza with 60% ethanol solution to obtain the alcohol extract, concentrate the ethanol to the maximum, adjust the pH value to 4.0, 125°C temperature, and a gauge pressure of 0.14MPa, and heat for 2 hours; the solution is filtered, and the filtrate is passed through D101 macroporous resin Column chromatography separation, first eluted with water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com