Establishment method of traditional Chinese medicine composite fingerprint
A technique for fingerprinting and establishing methods, which is applied in the field of quality control of traditional Chinese medicine compositions, can solve problems such as the inability to quantitatively analyze product components, the inability to guarantee the quality stability of medicinal materials, and the batch-to-batch quality uniformity of products to achieve good precision and quality. The effect of more standard and comprehensive quality standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Quality Control Method for Weilikang Granules Based on Fingerprint
[0054] Instruments: Agilent1260 high performance liquid chromatography, AX205 one hundred thousandth balance, Milli-Q ultrapure water preparation instrument.
[0055] Test drugs: paeoniflorin reference substance (lot number: MUST-12113099), naringin reference substance (lot number: 110722-201312), rutin reference substance (lot number: 100080-200707), emodin-8-O-β-D Glucopyranoside (batch number: W14-7-0): the above reference substances were purchased from China Institute for the Control of Pharmaceutical and Biological Products; glycyrrhizic acid (batch number: 20121098) reference substance, rosmarinic acid (batch number: 20140722) reference substance, salvianol Acid B reference substance (batch number: 20121033): purchased from Tianjin Yifang Technology Co., Ltd., methanol, formic acid (chromatographically pure), Weilikang granules (batch number 1304011, purchased from Sichuan Luye Baoguan...
Embodiment 2
[0067] Example 2: Establishment of fingerprints of Weilikang Granules
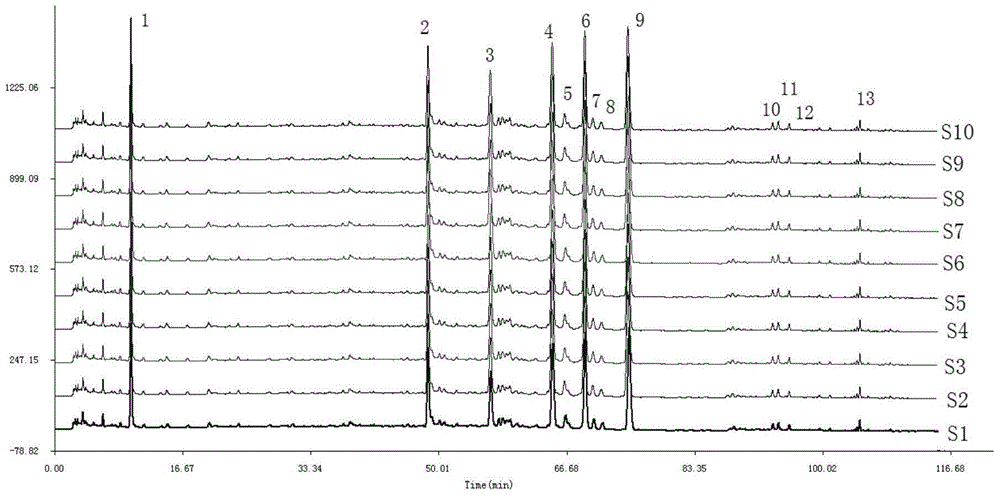
[0068] A total of 10 batches of Weilikang granules (1304011, 1304021, 1304031, 1304041, 1304051, 1304061, 1303151, 1303161, 1303171, 1303181) were determined by the method described in Example 1.
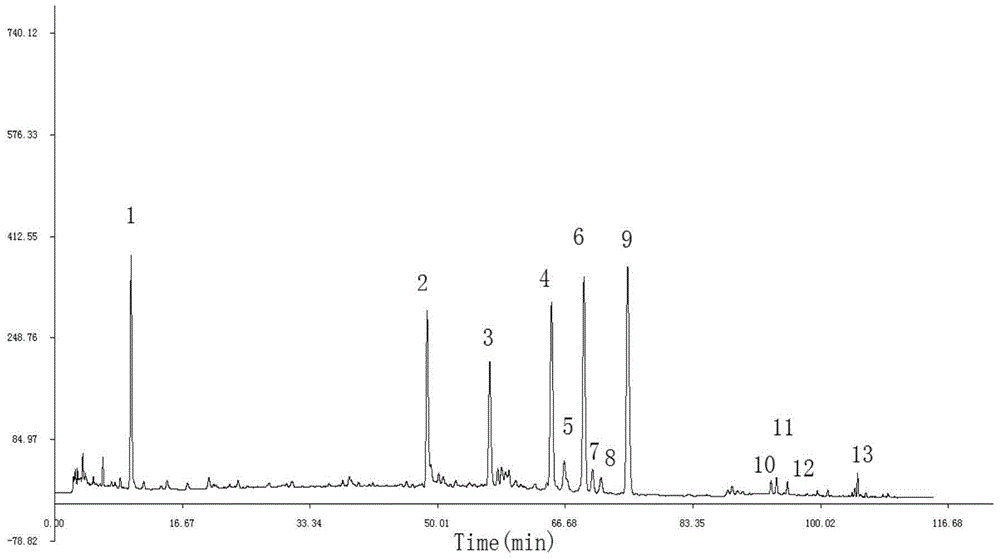
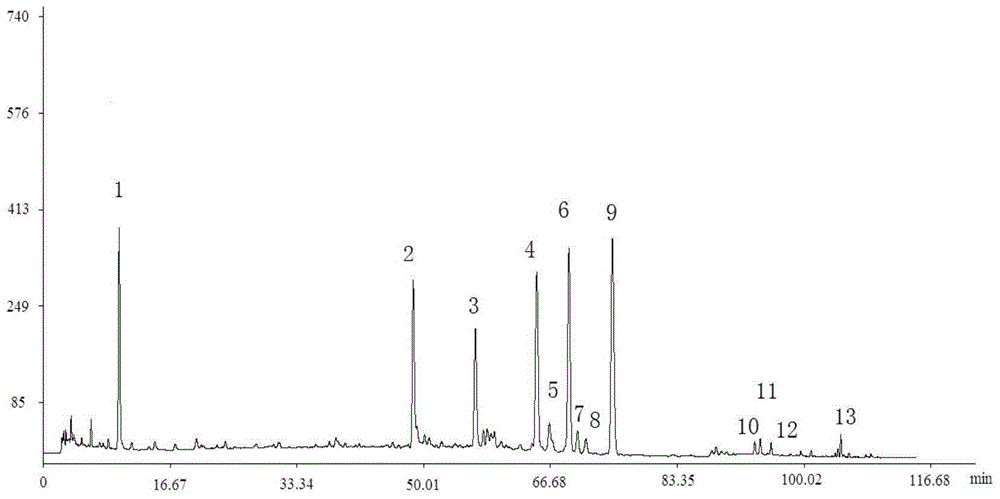
[0069] The fingerprints of 10 batches of Weilikang granules are as follows: Figure 4 shown.
[0070] Through the comparison of the HPLC spectra of 10 batches of Weilikang granules, the similarity evaluation was carried out: the "Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System 2004A Edition" was adopted by the National Pharmacopoeia Commission. Calculate the similarity of 10 batches of Weilikang granules, and the similarity between the 10 batches of Weilikang granules and the standard fingerprint (the common pattern S0 generated by 10 batches of Weilikang granules) is greater than 0.99. For specific results, see Table 8 shows.
[0071] Table 8 Similarity Comparison Results of Weilika...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com