Use of salvianolic acid B and its salt in treating parkinson's disease
A technology for Parkinson's disease and salvianolic acid, applied to salvianolic acid B and its salts in the field of treating Parkinson's disease, can solve problems such as inability to achieve therapeutic effects, inconvenience in use, etc., and achieve improved cerebral blood flow and safety. The effect of high sex and improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] [Example 1] Salvianolic acid B cytotoxicity detection
[0025] Materials and Methods
[0026] 1. SH-SY5Y cell culture
[0027] SH-SY5Y cells were cultured in a DMEM medium containing 10% fetal bovine serum (Gibco), 100 IU / ml penicillin, and 100 g / ml streptomycin at 37°C in a 5% CO2 incubator. Observe that the cells are in a good growth state. After culturing to the logarithmic growth phase, inoculate SH-SY5Y cells in a 96-well plate, 2×105 cells / well, and incubate at 37°C, 5% CO2 for 24 hours, until 80-90% of the cells are confluent Afterwards, the dosing experiment was carried out.
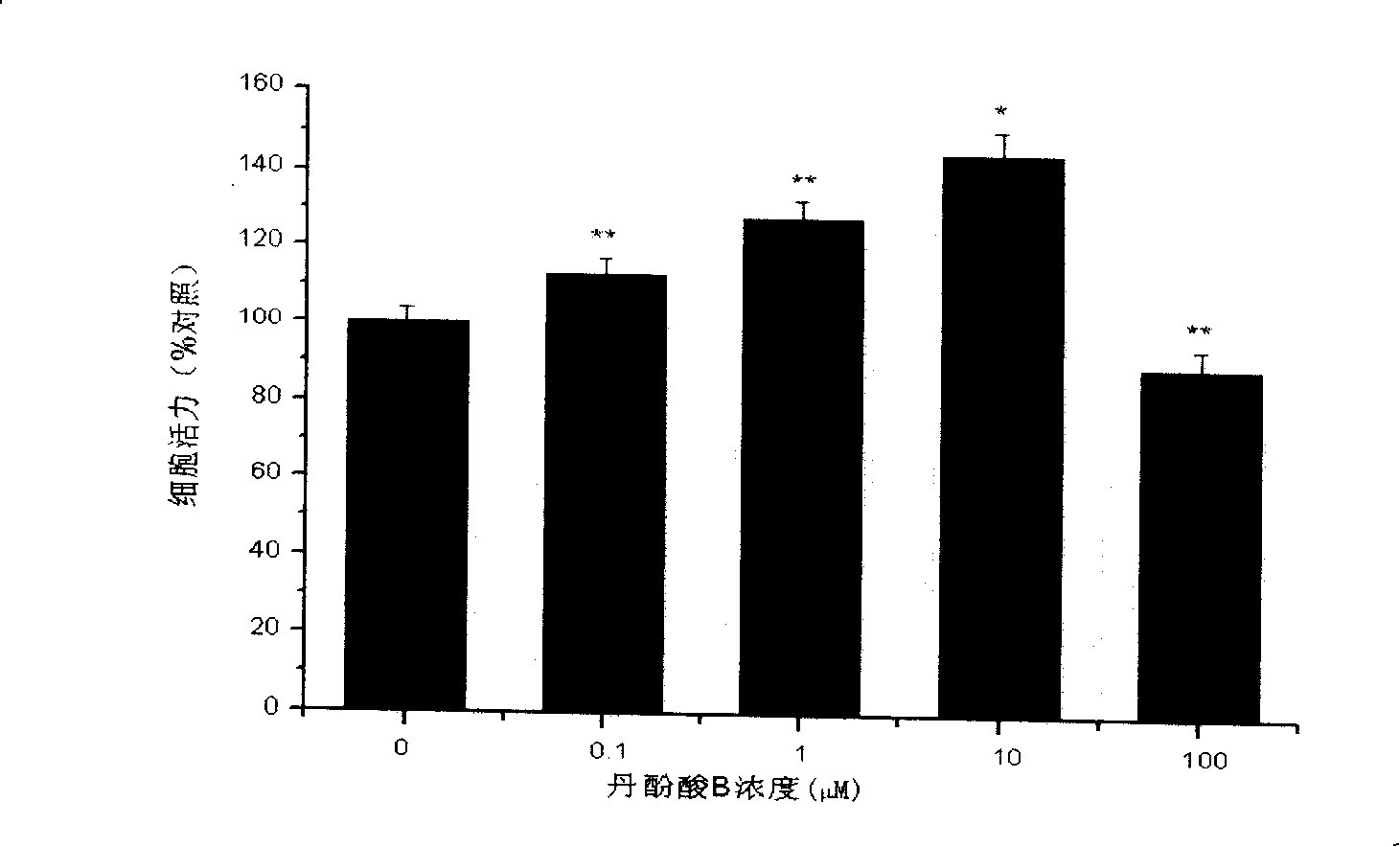
[0028] 2. Effect of salvianolic acid B on the proliferation of SH-SY5Y cells
[0029] SH-SY5Y cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco), 100 IU / ml penicillin, and 100 g / ml in a 37°C, 5% CO2 incubator. Observe that the cells are in a good growth state. After culturing to the logarithmic growth phase, inoculate SH-SY5Y cells in a 96-well plate, 2×105 ce...
Embodiment 2
[0036] [Example 2] The effect of salvianolic acid B on the loss of viability of SH-SY5Y cells caused by 6-hydroxydopamine (6-OHDA)
[0037] Materials and Methods
[0038] 1. SH-SY5Y cell culture
[0039] Method sees embodiment one
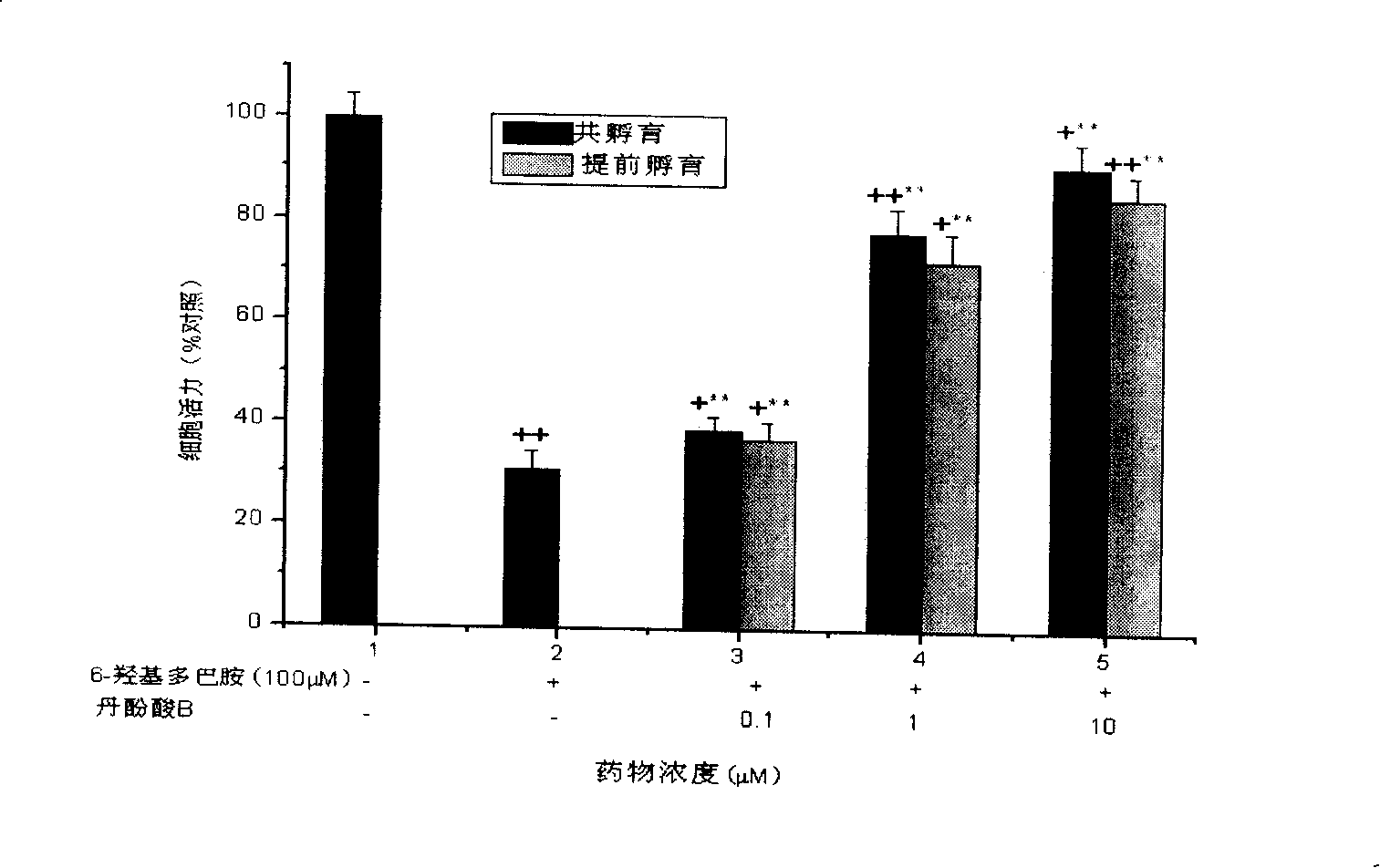
[0040] 2. Detection of the effect of salvianolic acid B on the loss of viability of SH-SY5Y cells induced by 6-hydroxydopamine (6-OHDA)
[0041] SH-SY5Y cells were cultured in DMEM medium containing 10% fetal bovine serum (Gibco), 100 IU / ml penicillin, and 100 g / ml in a 37°C, 5% CO2 incubator. Observe that the cells are in a good growth state. After culturing to the logarithmic growth phase, inoculate SH-SY5Y cells in a 96-well plate, 2×105 cells / well, and incubate at 37°C, 5% CO2 for 24 hours, until 80-90% of the cells are confluent Afterwards, one of the groups administered the compound salvianolic acid B at a final concentration of 2.5 μM / L, 5 μM / L, and 10 μM / L, respectively, with 3 wells for each concentration, and then administered 6-hydrox...
Embodiment 3
[0048] [Example 3] Detection of the impact of salvianolic acid B on striatal dopamine content in Parkinson's disease rat model
[0049] Materials and Methods
[0050] 1. SD rat culture
[0051]40 healthy adult male SD rats, weighing between 200 and 300 g, were selected and provided by the Animal Experiment Center of the Academy of Military Medical Sciences. Rats were kept at an ambient temperature of 25°C.
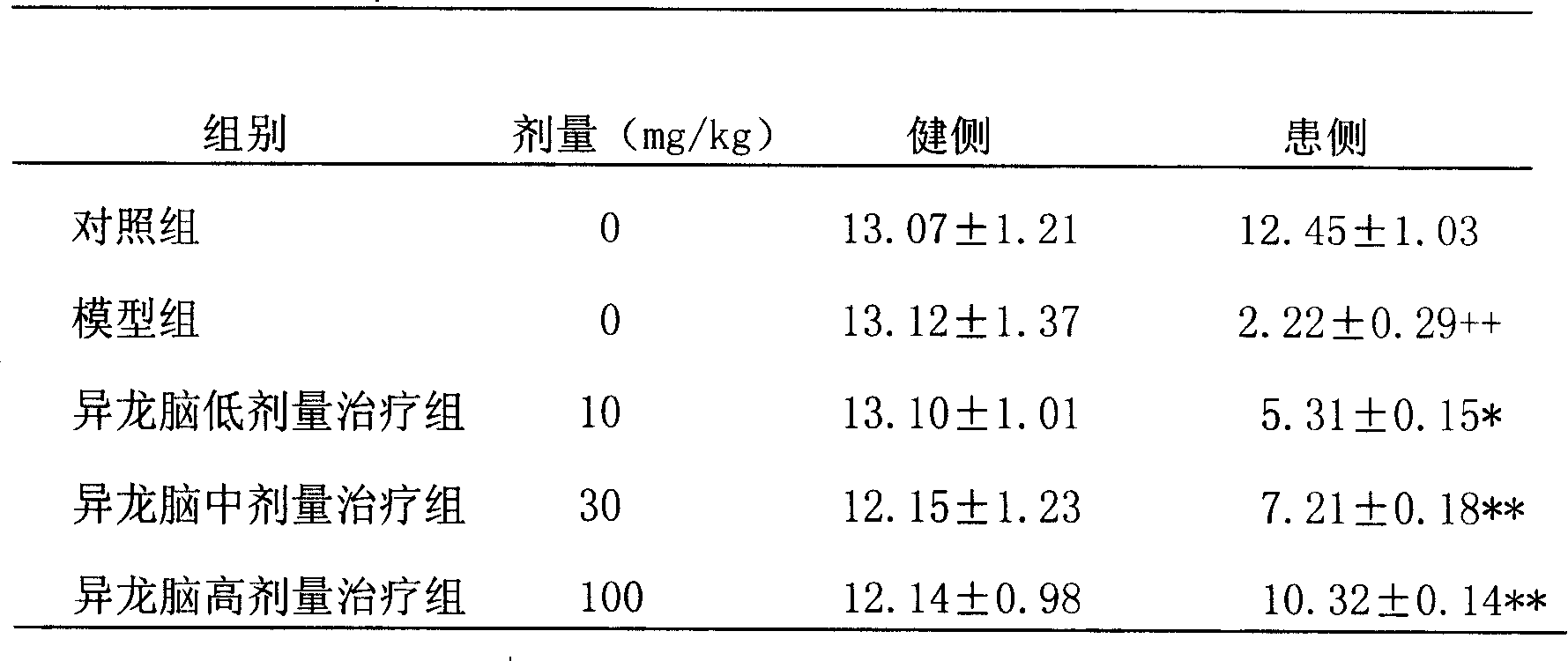
[0052] 2. Detection of the effect of salvianolic acid B on striatal dopamine content in Parkinson's disease rat model
[0053] Parkinson's rat model production: directional injection of micro-6-OHDA method: intraperitoneal injection of 3% (ω) pentobarbital sodium (30mg / kg) to anesthetize the rat, and fix the head of the rat on the brain stereotaxic apparatus , The anterior and posterior fontanelles are even, exposing the skull and cleaning the bone surface. According to the Paxinos and Watson rat brain stereotaxic atlas, the coordinates of the substantia nigra compact ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com