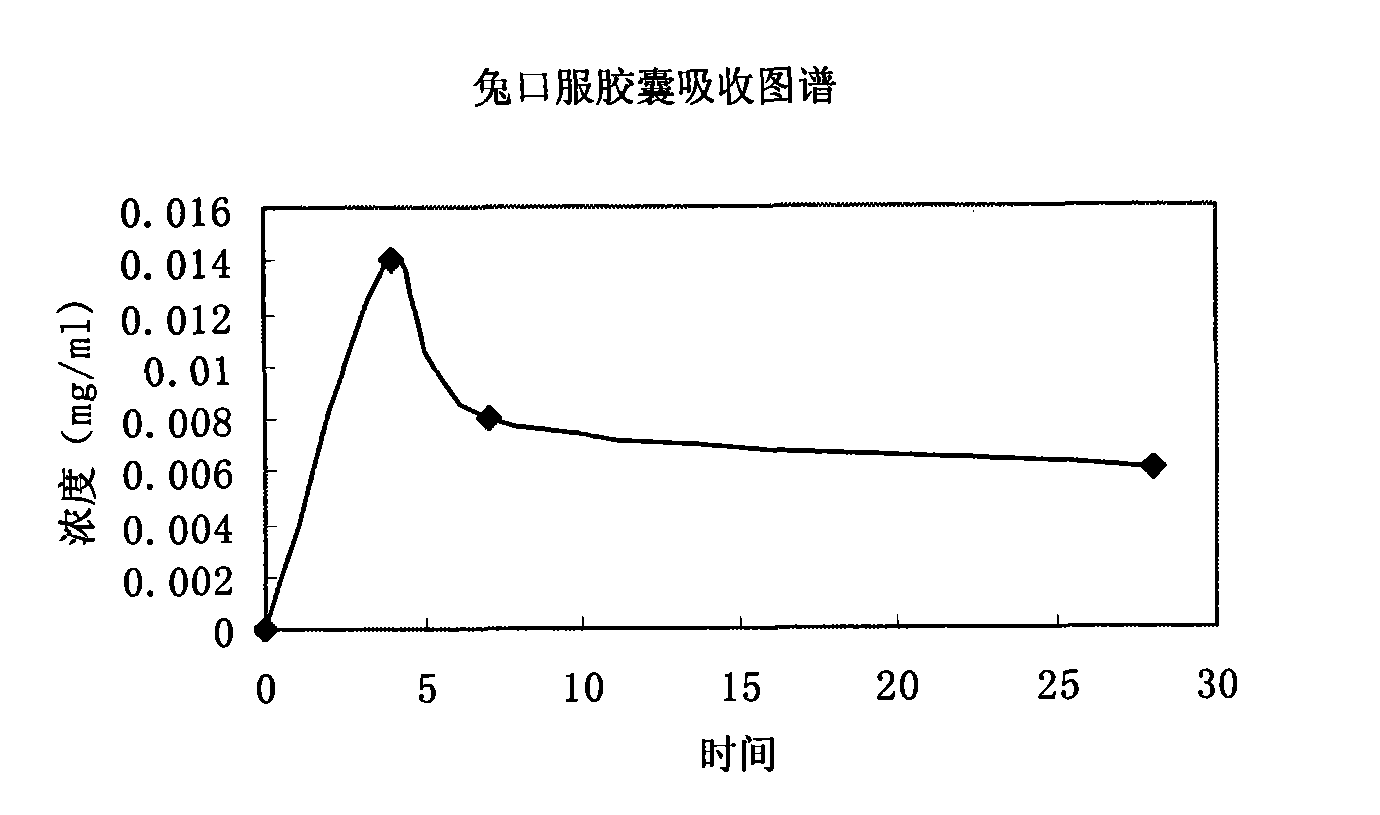
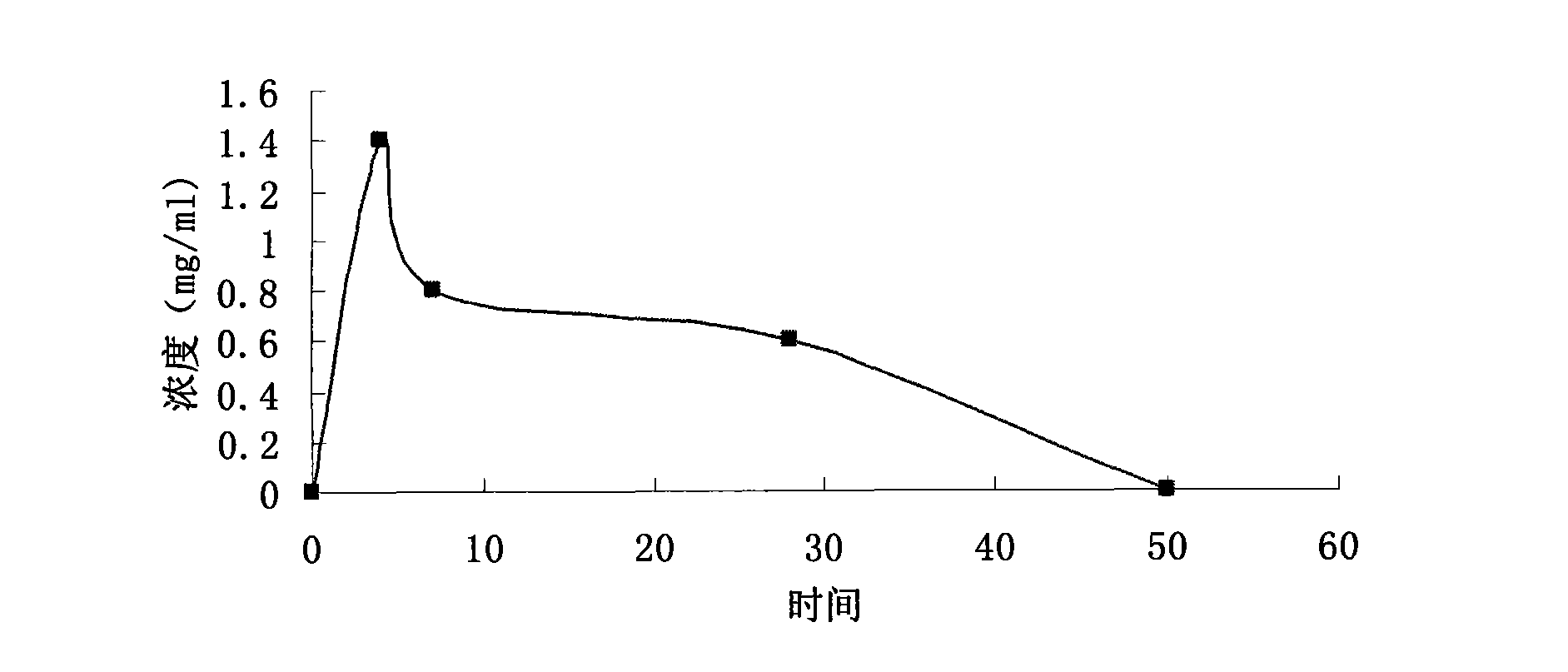
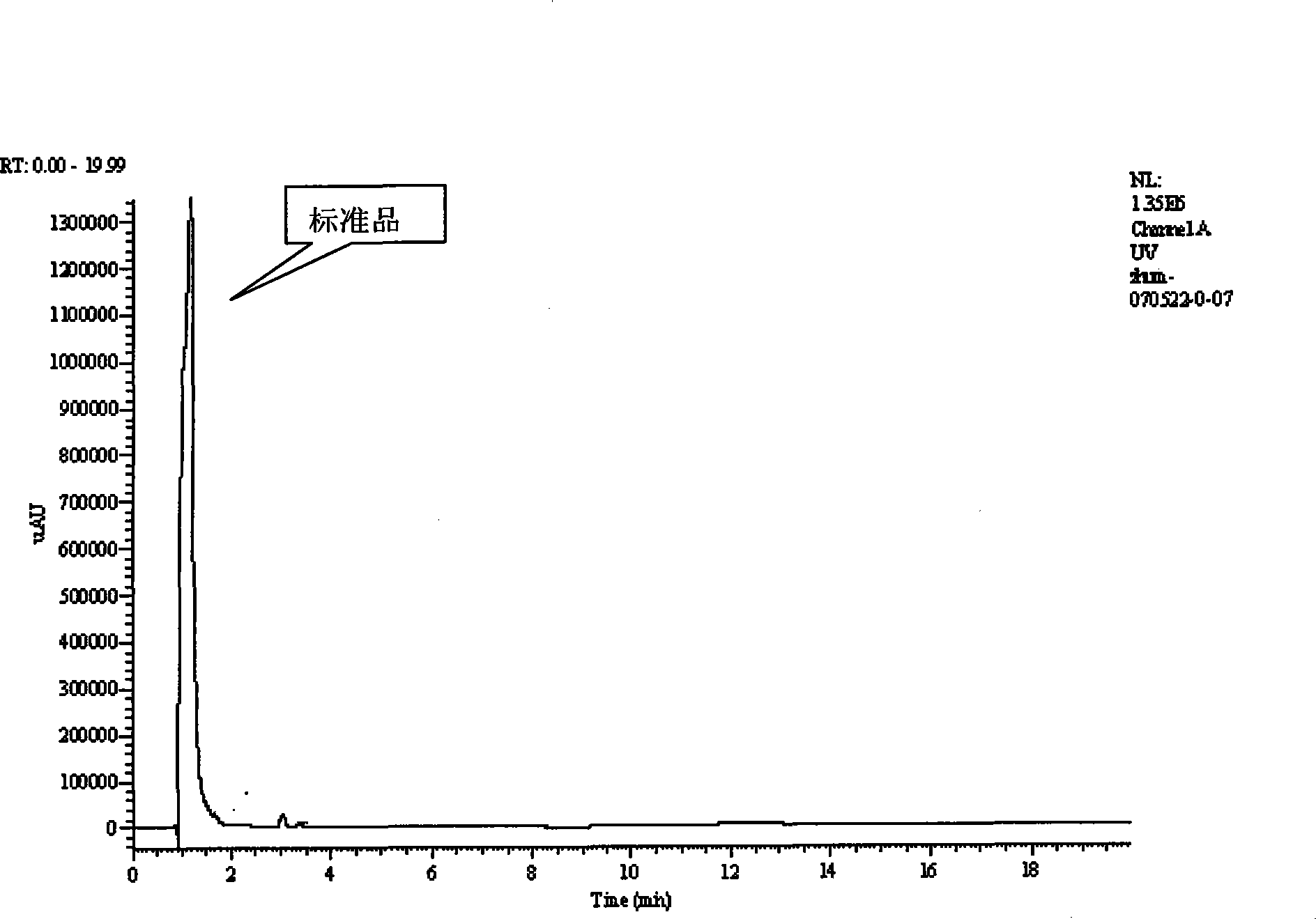
Calciparine/sodium salt nano oral preparation and preparation technique thereof
The technology of oral preparation and heparin calcium is applied in the field of heparin calcium/sodium salt nano oral preparation and its preparation technology. The effect of good intestinal absorption rate, good stability and long metabolism time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of low molecular weight heparin calcium nanoliposomes
[0029] Chitosan 1g is dissolved in the acetic acid solution 50mL of 0.1% concentration (w / w), obtains chitosan solution, filters, gets filtrate, and in the filtrate drips concentration is 1% (w / w) under magnetic stirring. Low-molecular-weight heparin calcium (Changzhou Tianpu Pharmaceutical Co., Ltd., batch number H1999007) aqueous solution until a milky white precipitate appears, the resulting suspension is centrifuged, and the precipitate is freeze-dried to obtain low-molecular-weight heparin calcium nanoliposomes. The encapsulation efficiency is as high as 98% (97.94±1.33%).
Embodiment 2
[0030] Embodiment 2: Preparation of low-molecular-weight heparin calcium nanoliposomes
[0031] Chitosan 1g is dissolved in the acetic acid solution 50mL of 2% concentration, obtains the chitosan solution of acidification, filters, gets filtrate, and in the filtrate, drips the low molecular weight heparin calcium (Changzhou Tiantian) that concentration is 20% in the filtrate under magnetic stirring General Pharmaceutical Co., Ltd., batch number H1999007) aqueous solution until a milky white precipitate appears, the resulting suspension is centrifuged, and the precipitate is freeze-dried to obtain low-molecular-weight heparin calcium nanoliposomes, and its nano-encapsulation efficiency is as high as 98% (98.06 ±1.10%).
Embodiment 3
[0032] Embodiment 3: Preparation of low molecular weight heparin calcium nanocapsules
[0033] The low-molecular-weight heparin calcium nanoliposomes obtained by the method in Example 1 were evenly divided into 20 enteric-coated capsules to obtain the low-molecular-weight heparin calcium nanocapsules, with a drug content of 50 mg / capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com