VEGF slowly releasing injection microsphere support and its preparation and use
A microsphere, sustained-release technology, applied in the field of medicine, can solve the problems of short half-life, fast degradation, and limited clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of VEGF slow-release microsphere scaffold by w / o / w solvent evaporation method
[0030] Dissolve 100 mg of PLGA (PLA:PGA=75:25, Mw=10,000) in 3.2 ml of dichloromethane to make an oil phase, dissolve 3 μg of VEGF and 100 mg of ammonium bicarbonate in 1 ml of redistilled water (containing 3% trehalose, 5 % mannitol) to form the inner water phase, which is added to the above-mentioned oil phase, homogenized to form w / o colostrum, 120ml of 0.1% PVA solution is placed in a stirring vessel, and the colostrum is stirred (450rpm) Quickly add it into the external water phase to fully homogenize. After five minutes, reduce the speed to 200rpm and add 30ml of distilled water to the external water phase. Stir at room temperature for 4 hours. After the microspheres are hardened, filter, wash, and freeze-dry. After sealing and dispensing, it can be irradiated and sterilized. The particle size is about 500 μm.
Embodiment 2
[0031] Embodiment 2: The w / o / w solvent volatilization method that medicine is added in the form of micropowder prepares VEGF slow-release microsphere stent
[0032] Disperse 1.2 mg of PEG (PEG6000), 300 μg of VEGF and protective agent (600 μg of zinc carbonate) in 1 ml of double-distilled water, vortex and mix for about 3 minutes, subpackage, freeze-dry, wash with dichloromethane, centrifuge, remove PEG, and obtain VEGF Micronized. Dissolve 100 mg of PLGA (PLA:PGA=75:25, Mw=10000) in 3.2 ml of dichloromethane to make an oil phase, and dissolve 100 mg of ammonium bicarbonate in 1 ml of redistilled water (containing 3% trehalose, 5% manna Alcohol) to form the inner water phase, add it to the above oil phase, homogenize for three minutes, add micronized medicine and homogenize at a low speed for one minute to form w / o colostrum, put 120ml of 0.1% PVA solution in In a stirring container, quickly add colostrum into the external water phase under stirring (450rpm) to fully homogeni...
Embodiment 3
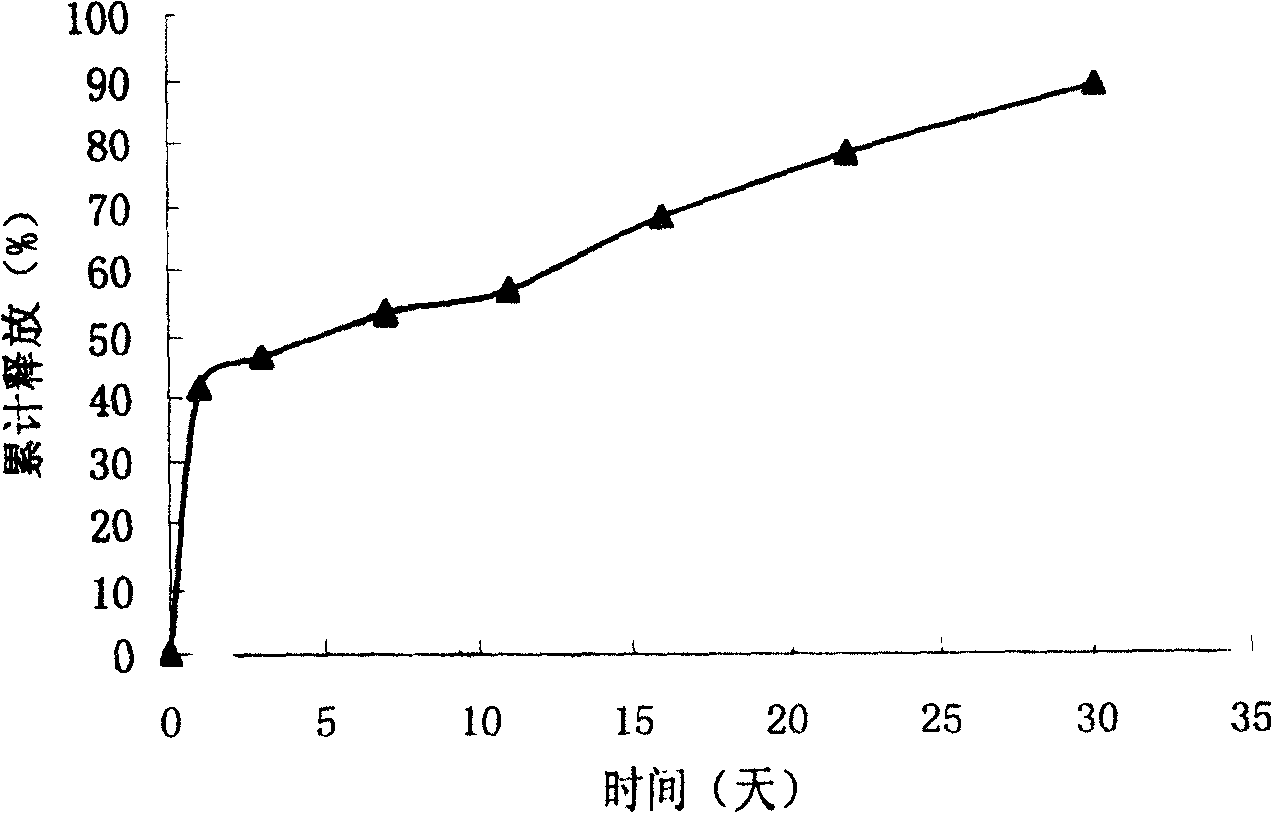
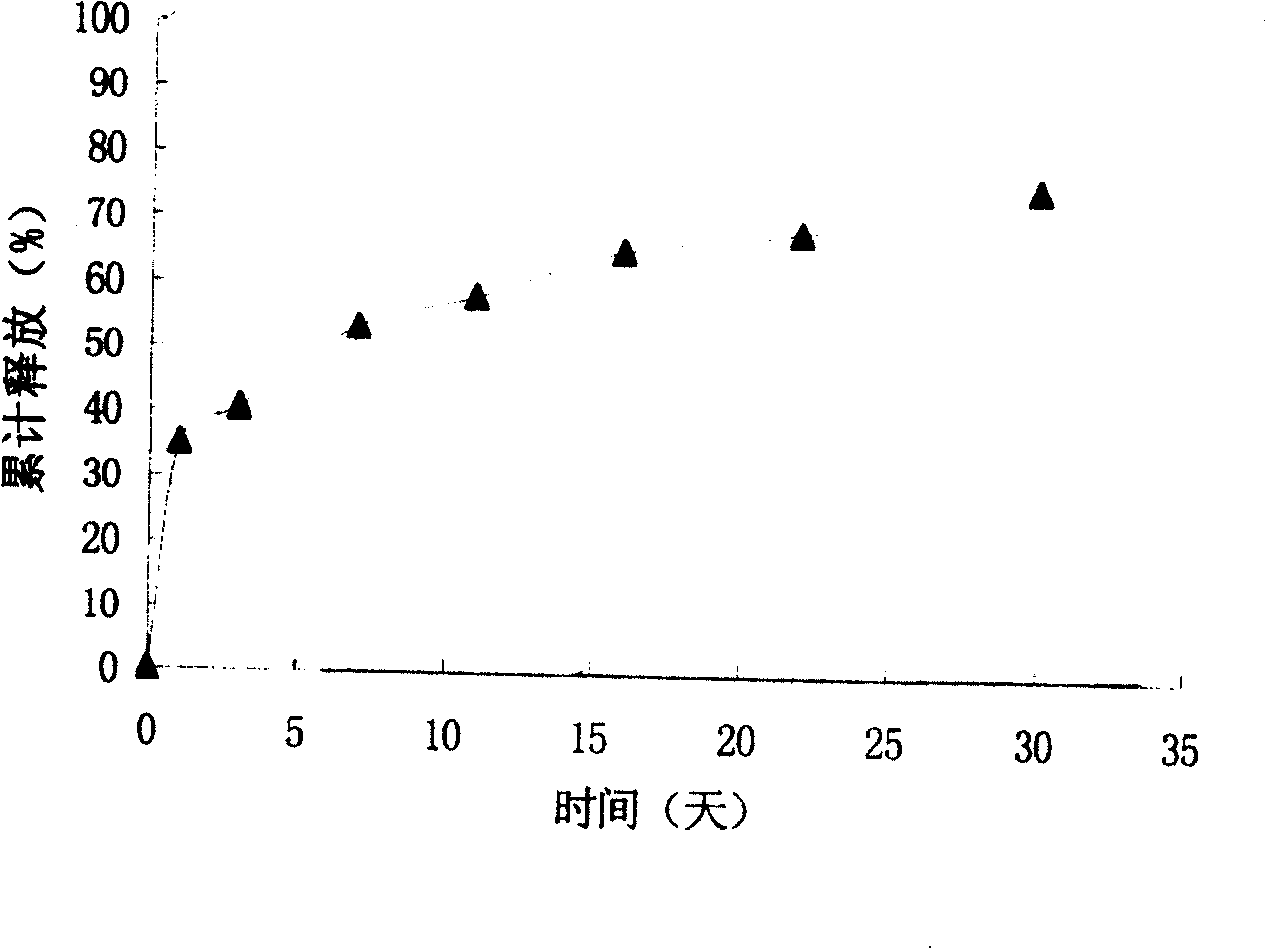
[0033] Embodiment 3: VEGF sustained-release microspheres are tested through in vitro release
[0034] Instruments: 0508-2 desktop low-speed centrifuge (Shanghai Medical Instrument Co., Ltd.); XW-80 vortex mixer (Shanghai First Medical College Instrument Factory); CARY 100 UV spectrophotometer (Varian, USA); CS501 super constant temperature Water bath (Shanghai Pudong Rongfeng Scientific Instrument Co., Ltd.), FA1004 1 / 10,000 electronic balance (Shanghai Balance Instrument Factory);
[0035] Method and operation: Accurately weigh about 20 mg of drug-containing microspheres or blank microspheres and place them in a 7ml centrifuge tube, add 1.5ml of 10mM pH 7.2 phosphate buffer (containing 0.02% sodium azide as a bacteriostatic agent, 0.02% F -68 as a wetting agent), placed in a constant temperature water bath shaker at 37°C, with an oscillation speed of 100rpm. Take out the centrifuge tubes on day 1 and day 3 respectively, place them in a centrifuge at 2000rpm for 5min, carefully...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com