Method for detecting skin permeability of metronidazole gel
A metronidazole gel and metronidazole technology, applied in measuring devices, material inspection products, testing pharmaceutical preparations, etc., can solve problems such as poor patient tolerance, large side effects, prone to anaphylactic shock and nervous system diseases , to achieve the effect of high accuracy and convenient detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
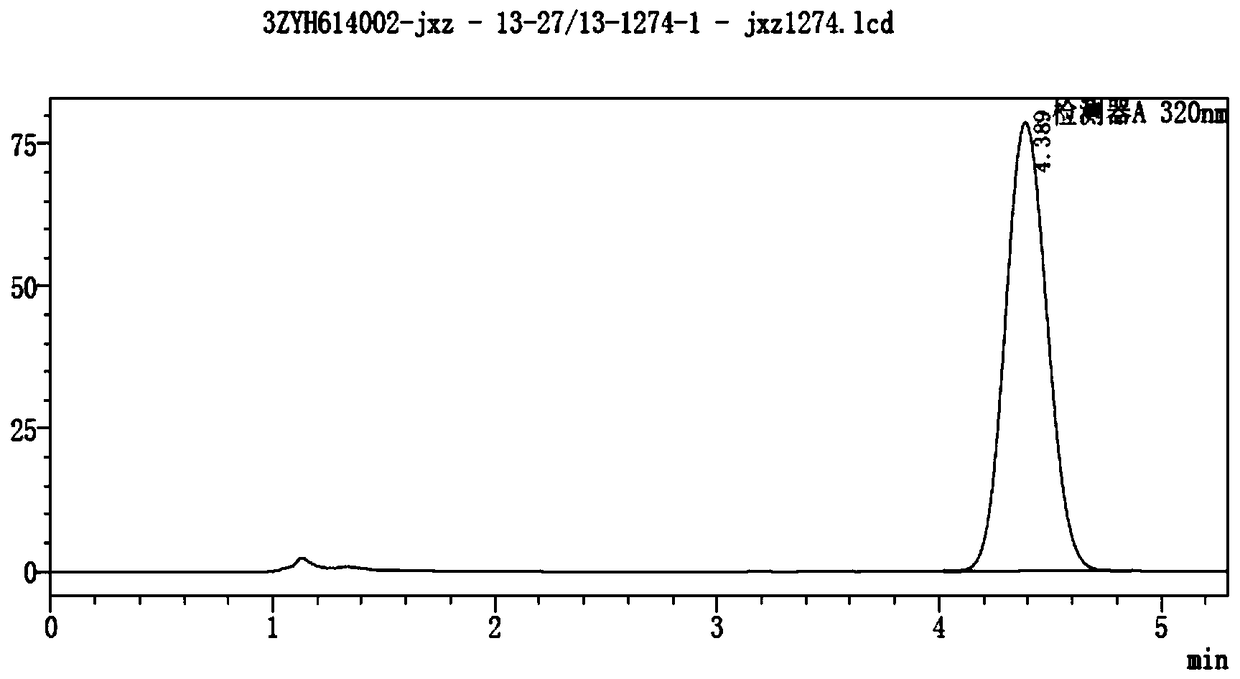
[0040] Take the prescription screening sample (batch number: 20161201) and the marketed original research sample (batch number: KAAU) produced by Zhuzhou Qianjin Pharmaceutical Co., Ltd., and use the artificial skin membrane to conduct the test according to the following in vitro skin penetration test method:
[0041] (1) Set the release area to 0.6cm 2 The artificial skin film is fixed at one end of the release pool of the transdermal absorption instrument, the rough surface of the artificial skin film faces the release pool, and 0.15 g of metronidazole gel test product is evenly coated on the rough surface of the artificial skin film ;
[0042] (2) adjust the temperature of the water in the heat preservation tank of the transdermal tester to be constant at 32°C, and turn on the magnetic stirring; the release pool is fixed on the receiving pool, and the 0.9% sodium chloride solution of 5ml is added as the receiving liquid in the receiving pool, Ensure that the side of the ar...
Embodiment 2
[0052] The prescription screening sample (batch number: 20170410) and the marketed original research sample (batch number: KAAU) produced by Zhuzhou Qianjin Pharmaceutical Co., Ltd. were taken, and the same method as in Example 1 was used for testing.
[0053] The results are shown in Table 2.
[0054] Table 2: Determination results of cumulative drug penetration of self-developed samples and original marketed samples (n=6)
[0055]
[0056] Conclusion: With time (h) as the abscissa and permeation (μg) as the ordinate, linear fitting is performed, and the R of the self-developed preparation (20170410 batches) and the reference preparation 2 The values are all greater than 0.98, conforming to the zero-order transdermal law, and the slope of the self-developed preparation is similar to that of the reference preparation, indicating that the self-developed preparation is equivalent to the skin penetration performance of the reference preparation.
Embodiment 3
[0058] The prescription screening sample (batch number: 20170414) and the marketed original research sample (batch number: KAAU) produced by Zhuzhou Qianjin Pharmaceutical Co., Ltd. were taken, and the same method as in Example 1 was used for testing.
[0059] The results are shown in Table 3.
[0060] Table 3: Determination results of cumulative drug penetration of self-developed samples and original marketed samples (n=6)
[0061]
[0062] Conclusion: With time (h) as the abscissa and permeation (μg) as the ordinate, linear fitting is performed, and the R of the self-developed preparation (batch number 20170414) and the reference preparation 2 The values are all greater than 0.98, conforming to the zero-order transdermal law, and the slope of the self-developed preparation is similar to that of the reference preparation, indicating that the self-developed preparation is equivalent to the skin penetration performance of the reference preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com