Preparation method for controlled release preparation, especial for zero-order release controlled release preparation
A technology for controlled release preparations and drug release, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Drug rate drop and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Each basic step in the preparation method of the controlled-release preparation is described in detail below.
[0030] Firstly, the most important step 3) of the present invention, the healing (curing) treatment coating film is described.
[0031] In order to improve the stability of the drug release of the preparation, it is necessary to heal the above-mentioned coating film to eliminate the numerous extremely small micropores produced in the coating process in the coating film and form a dense coating film to ensure the relative stability of drug release.
[0032] After the coating is finished, the solvent or dispersant of the polymer in the coating film has basically volatilized, leaving many extremely small micropores in the coating film, and the polymer particles in the coating film are often not completely fused. It is believed that under the action of the micropore additional pressure (ΔP) generated by the interfacial tension between the polymer and the air, thes...
Embodiment 1
[0091] Prepare samples and control samples according to the following prescriptions and processes
[0092] 1), preparation of tablet core:
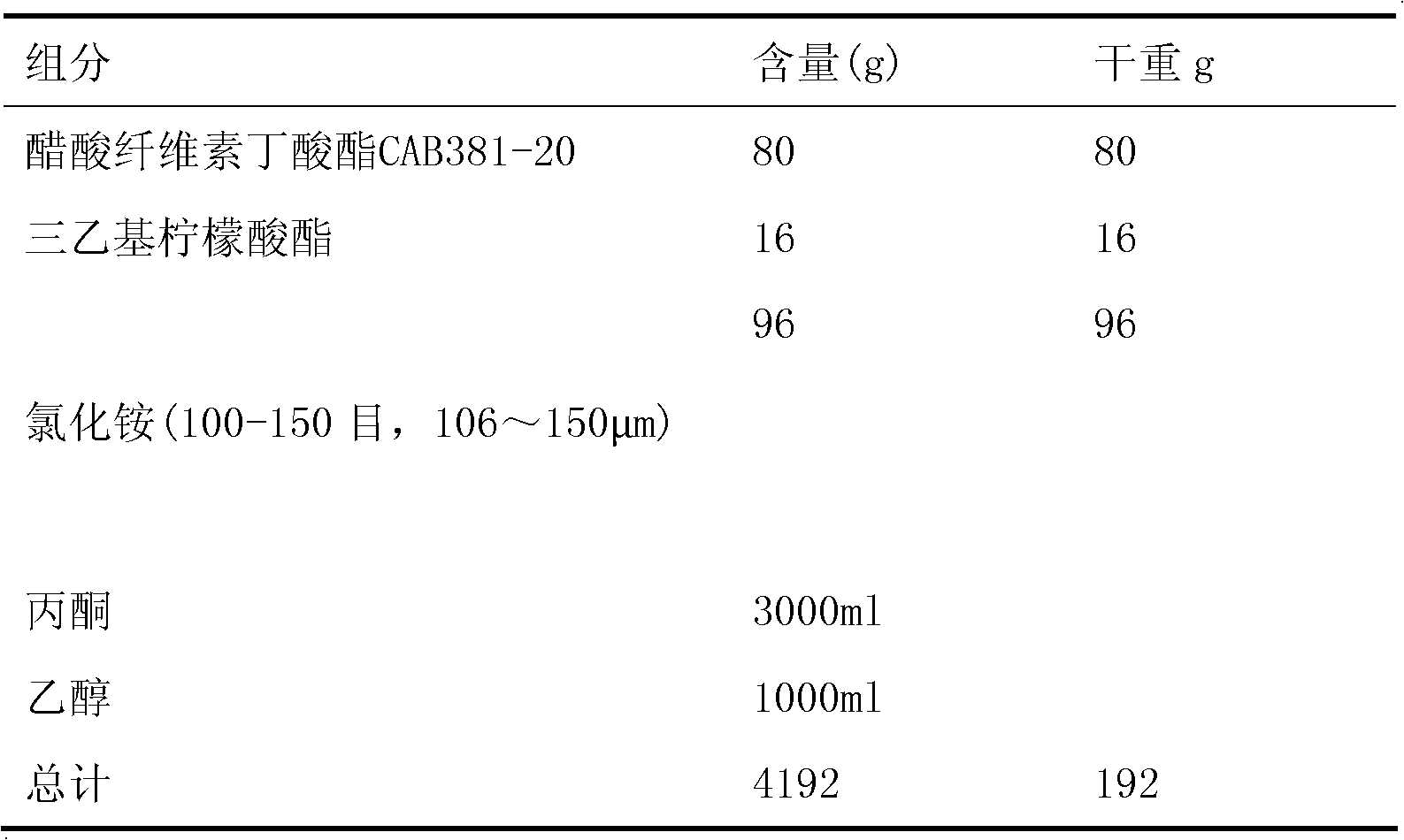
[0093]
[0094]
[0095] Mix simvastatin, carbopol, ground and 200-mesh sieved sodium citrate, lactose and polyvinylpyrrolidone, and use 10% (by weight) ethanol solution containing water (containing the required BHA) to prepare grain. Pass the wet material through a 18 mesh sieve and dry overnight, granulate, lubricate with magnesium stearate, mix well, and compress the homogeneous mixture with a 1 / 4 inch standard concave round tool, using a compression force of 1000 lbs. . The thickness of the compressed tablet is 3.89 mm, and the hardness is 8-10 kg.
[0096] 2), the tablet core is covered with a water-soluble film coat
[0097] Wrap a water-soluble film coat on the above tablet core. The coating material for water-soluble film coating was an aqueous solution containing 4.5% hydroxypropylmethylcellulose (Pharmacoat, 603 / ShinE...
Embodiment 2
[0119] Prepare samples and control samples according to the following prescriptions and processes
[0120] 1), preparation of tablet core:
[0121]
[0122] Glipizide, polyethylene oxide and sodium chloride are mixed, then mixed with magnesium stearate and then molded into a 502mg tablet core. A 12mm standard concave circular die is used to compress the tablet. The compression force used 1200~1800kg, pressing time 1~2s, 6~8kg.
[0123] 2), preparation of coating solution:
[0124] Add cellulose acetate to ethyl acetate-ethanol (95:5) to obtain a 5% solution as the oil phase, and use a 3 mg / ml sodium lauryl sulfate aqueous solution as the water phase; Slowly add the water phase to the oil phase at a speed of not less than 3000 rpm to form a W / O emulsion, and continue to add until O / W colostrum is formed. Pass the colostrum through a high-pressure homogenizer for 6 times. The organic solvent was removed from the resulting emulsion using a rotary evaporator at 40°C under red...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com