Felodipine controlled-release preparation
The technology of a preparation, lodipine, is applied in the field of felodipine controlled-release preparations, which can solve the problems of organ damage, frequent drug administration, and large blood pressure fluctuations, achieve stable blood drug concentration, reduce drug administration times, improve safety and effect of effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Chip core 1000 pieces
[0038] Felodipine 5g
[0040] Starch 15g
[0041] Polyethylene glycol 10g
[0042] Magnesium stearate 0.5g
[0043] 5% starch paste appropriate amount
[0044] Coating solution (100ml)
[0045] Cellulose acetate 20g
[0046] Polyethylene glycol 0.5g
[0047] Appropriate amount of distilled water
[0048] The preparation method of this tablet is as follows:
[0049] 1, Felodipine is pulverized to 200 orders, sieves, adds starch and mixes;
[0050] II, sodium chloride, polyethylene glycol, magnesium stearate are pulverized to 100 orders respectively, sieve, mix with the mixture that I makes;
[0051] III, adding 5% starch paste to the mixture prepared by II to make soft material in an appropriate amount;
[0052] IV. Dried at 50-60°C, granulated and compressed into tablets; made into each tablet containing 5 mg of felodipine.
[0053] V. Coat the tablet prepared by IV, and punch a small hole of 10-900 μm on ...
Embodiment 2
[0060]Chip core 1000 pieces
[0061] Felodipine 10g
[0062] Sucrose 80g
[0063] Mannitol 15g
[0064] Polyethylene glycol 10g
[0065] Magnesium stearate 0.5g
[0066] 30% syrup appropriate amount
[0067] Coating liquid is the same as embodiment 1
[0068] The preparation method of this tablet is as follows:
[0069] 1, Felodipine is pulverized to 200 orders, sieves;
[0070] II, sucrose, mannitol, Polyethylene Glycol, magnesium stearate are pulverized to 100 orders respectively, sieve, mix with the felodipine that I makes;
[0071] III, adding 30% syrup to the mixture prepared by II to make soft material in an appropriate amount;
[0072] IV, 50~60 ℃ of drying, whole grain, tabletting, make the tablet that each contains 10mg felodipine.
[0073] V. Coat the tablet prepared by IV, and punch a small hole of 10-900 μm on one side of the tablet with a laser after passing the test.
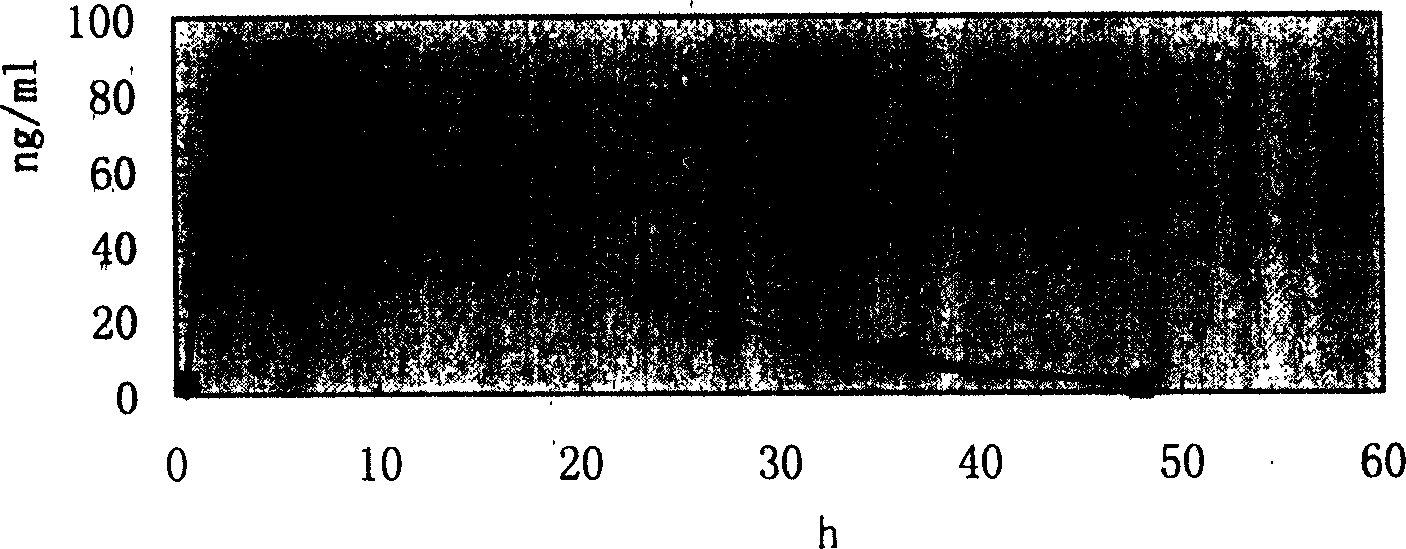
[0074] The release situation of embodiment 2 felodipine is shown in Table 2:
[0075...
Embodiment 3
[0081] Chip core 1000 pieces
[0082] Felodipine 5g
[0084] Polyethylene glycol 10g
[0085] Hypromellose 10g
[0086] Starch 15g
[0087] Magnesium stearate 0.5g
[0088] 5% starch paste appropriate amount
[0089] Coating liquid is the same as embodiment 1
[0090] The preparation method of this tablet is as follows:
[0091] 1, Felodipine is pulverized to 200 orders, sieves, adds starch and mixes;
[0092] II, sodium chloride is crushed to 100 mesh, sieved, mixed with the mixture prepared by I and polyethylene glycol, hypromellose, magnesium stearate;
[0093] III, adding 5% starch paste to the mixture prepared by II to make soft material in an appropriate amount;
[0094] IV. Dried at 50-60°C, granulated and compressed into tablets to make each tablet containing 5 mg of felodipine.
[0095] V. Coat the tablet prepared by IV, and punch a small hole of 10-900 μm on one side of the tablet with a laser after passing the test.
[0096] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com