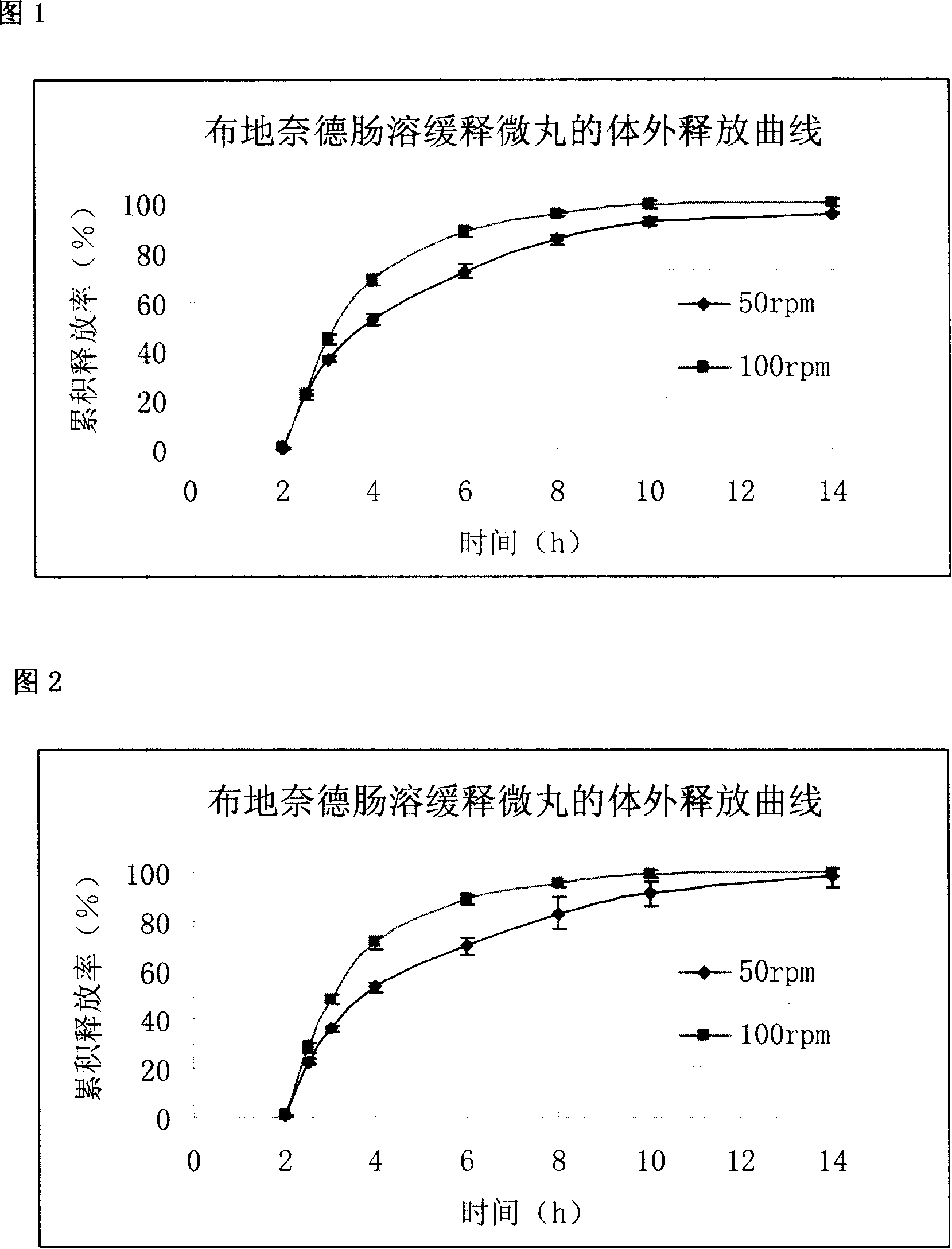
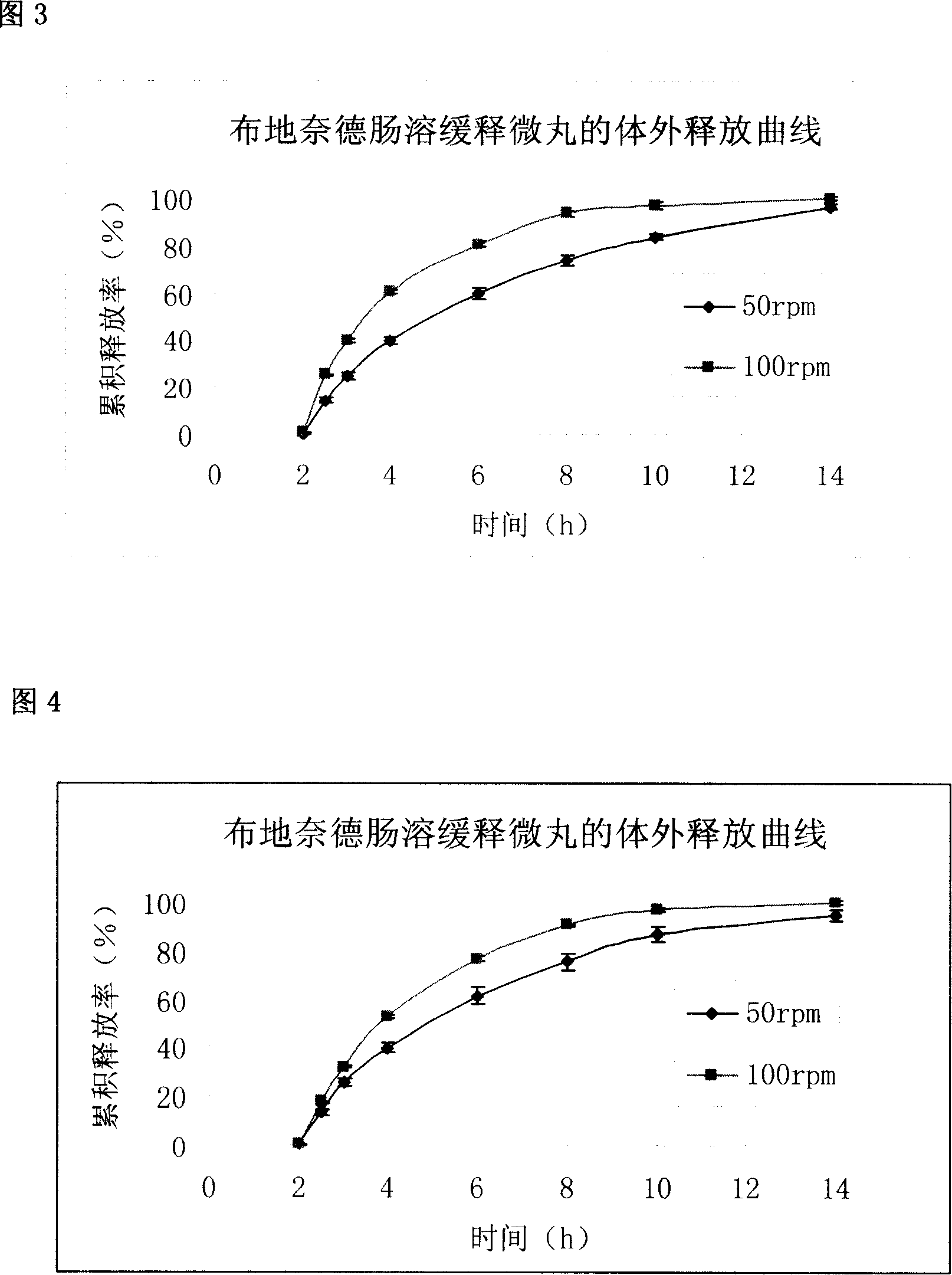
Budesonide intestines sustained release dextromethorphan pellets and method of manufacturing the same
A slow-release pellet and enteric-coated technology, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of high price, unknown clinical effect, and high cost, and achieve Effect of good film formation and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Prescription for Blank Ball Core
[0051] Microcrystalline Cellulose 80g
[0052] Starch 60g
[0053] Hypromellose 20g
[0054] Silica 20g
[0056] Drug Coating Solution Prescription
[0057] Blank ball core 80g
[0058] Budesonide 1g
[0059] Hypromellose 2g
[0060] 50% ethanol 50g
[0061] Sustained-release layer coating solution prescription
[0062] Drug-containing immediate-release pellet core 100g
[0063] Ethylcellulose 3g
[0064] Sodium Lauryl Sulfate 0.3g
[0065] Tributyl citrate 0.3
[0067] 95% ethanol 60g
[0068] Enteric layer coating liquid prescription
[0069] Sustained-release pellets 100g
[0070] Eudragit L100 16.5g
[0071] Triethyl citrate 1.65g
[0073] 95% ethanol 250g
[0074] According to the formula quantity of each layer above, mix the microcrystalline cellulose and starch with the prescription quantity of the blank ball core evenly, add ...
Embodiment 2
[0080] Prescription for Blank Ball Core
[0081] Lactose 100g
[0082] Starch 30g
[0083] Sucrose 40g
[0084] Talc powder 20g
[0086] Drug Coating Solution Prescription
[0087] Blank ball core 80g
[0088] Budesonide 1g
[0089] Polyvinylpyrrolidone 2g
[0090] 80% ethanol 50g
[0091] Sustained-release layer coating solution prescription
[0092] Drug-containing immediate-release pellet core 100g
[0093] Ethyl cellulose aqueous dispersion Surelease 8g (solid content is 2g)
[0094] Hypromellose 1g
[0095] water 10g
[0096] Enteric layer coating liquid prescription
[0097] Sustained-release pellets 100g
[0098] Eudragit L30D-55 55g (16.5g solids)
[0099] Triethyl citrate 1.65g
[0100] Talc powder 4.125g
[0101] water 50g
[0102] Mix the lactose and starch of the prescription amount of the blank ball core according to the above-mentioned formula quantity, add the adhesive prepared by sucrose, use a granulator or a c...
Embodiment 3
[0108] Drug-containing immediate-release pill core prescription
[0109] Lactose 90g
[0110] Starch 30g
[0111] Polyvinylpyrrolidone 40g
[0112] Talc powder 20g
[0113] Micronized Budesonide 3g
[0114] Sustained-release layer coating solution prescription
[0115] Drug-containing immediate-release pellet core 100g
[0116] Ethyl cellulose aqueous dispersion Aquacoat 15g (solid content 4.5g)
[0117] Tween 80 0.45g
[0118] water 15g
[0119] Enteric layer coating liquid prescription
[0120] Sustained-release pellets 100g
[0121] Eudragit S100 5.5g
[0122] Eudragit L100 11g
[0123] Polyethylene glycol 6000 1.65g
[0124] Talc powder 4.125g
[0125] 95% ethanol 250g
[0126] Mix the micronized drug with the prescribed amount of lactose, starch, polyvinylpyrrolidone, and talcum powder according to the above-mentioned formula quantities, and make pellets by rolling or extrusion-spheronization or centrifugal-fluidization Prepare the required micropills with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com