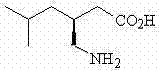
Pregabalin controlled release preparation, and preparation method thereof
A technology of pregabalin and controlled-release preparations, which is applied in the field of pregabalin controlled-release preparations for the treatment of neuropathic pain and its preparation, which can solve the problems of loss of curative effect, failure to reproduce multi-dose drug bioavailability, malabsorption, etc. , to achieve the effect of prolonging the residence time, prolonging the drug action time, and reducing the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Prepare 1000 pregabalin controlled-release preparations and adopt the following prescription:
[0036] Pregabalin 330g
[0037] Calcium hydrogen phosphate 103.5g
[0038] Carbomer 30g
[0039] Polycarbophil 30g
[0040] Hypromellose 3cp 30g
[0041] Sodium bicarbonate 60g
[0043] Micronized silica gel 1.5g
[0044] Its preparation method:
[0045] 1. Pass pregabalin pretreatment through an 80-mesh sieve, calcium hydrogen phosphate pass through an 80-mesh sieve, and sodium bicarbonate, magnesium stearate, and micropowder silica gel pass through an 80-mesh sieve respectively;
[0046] 2. Prepare 5% hydroxypropyl methylcellulose and 75% ethanol solution as a binder;
[0047] 3. Mix pregabalin, calcium hydrogen phosphate, carbomer, and polycarbophil, and use 5% hypromellose 75% ethanol solution to granulate;
[0048] 4. Granulate after drying the wet granules;
[0049] 5. Mix the granulated granules with additional auxi...
Embodiment 2
[0052] Embodiment 2: prepare 1000 pregabalin controlled-release tablets, adopt following prescription:
[0053] Pregabalin 330g
[0054] Microcrystalline Cellulose 102 123.5g
[0055] Carbomer 70g
[0056] Hypromellose 3cp 20g
[0057] Octadecanol 45g
[0059] Micronized silica gel 1.5g
[0060] Preparation method: 1. Pretreatment pregabalin is passed through an 80-mesh sieve, and stearyl alcohol, magnesium stearate, and micropowdered silica gel are respectively passed through an 80-mesh sieve;
[0061] 2. Pregabalin, microcrystalline cellulose, carbomer, hypromellose, stearyl alcohol. Micropowder silica gel is mixed, then mixed evenly with magnesium stearate;
[0062] 3. Perform direct compression according to the test results of the intermediate content to obtain pregabalin sustained-release tablets.
Embodiment 3
[0063] Embodiment 3: prepare 1000 pregabalin controlled-release tablets, adopt following prescription:
[0064] Pregabalin 330g
[0065] Lactose 87g
[0066] Sodium Alginate 80g
[0068] Polycarbophil 6g
[0069] Octadecanol 45g
[0070] Micronized silica gel 12g
[0071] Makes 1000 capsules
[0072] Its preparation method:
[0073] 1. Pass pregabalin pretreatment through an 80-mesh sieve, and lactose, stearyl alcohol, and micropowdered silica gel respectively pass through an 80-mesh sieve;
[0074] 2. Mix pregabalin, lactose, polycarbophil, sodium alginate, calcium chloride, stearyl alcohol, and micronized silica gel evenly;
[0075] 3. Perform direct compression according to the test results of the intermediate content to obtain pregabalin sustained-release tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com