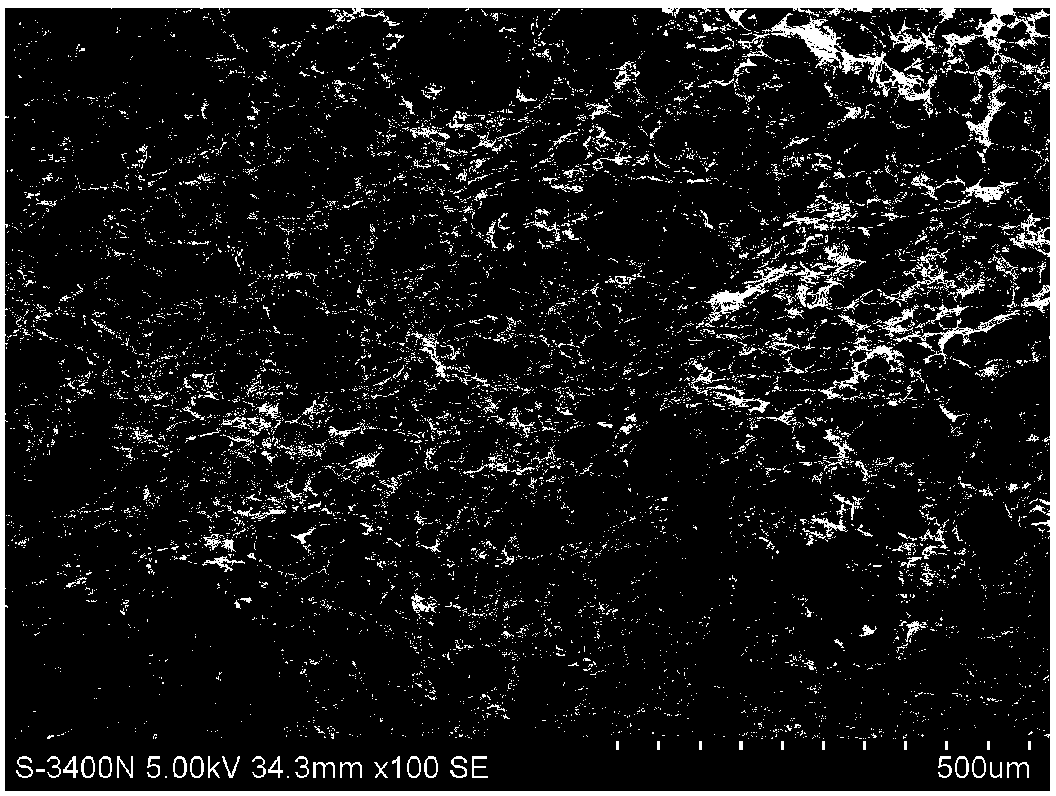
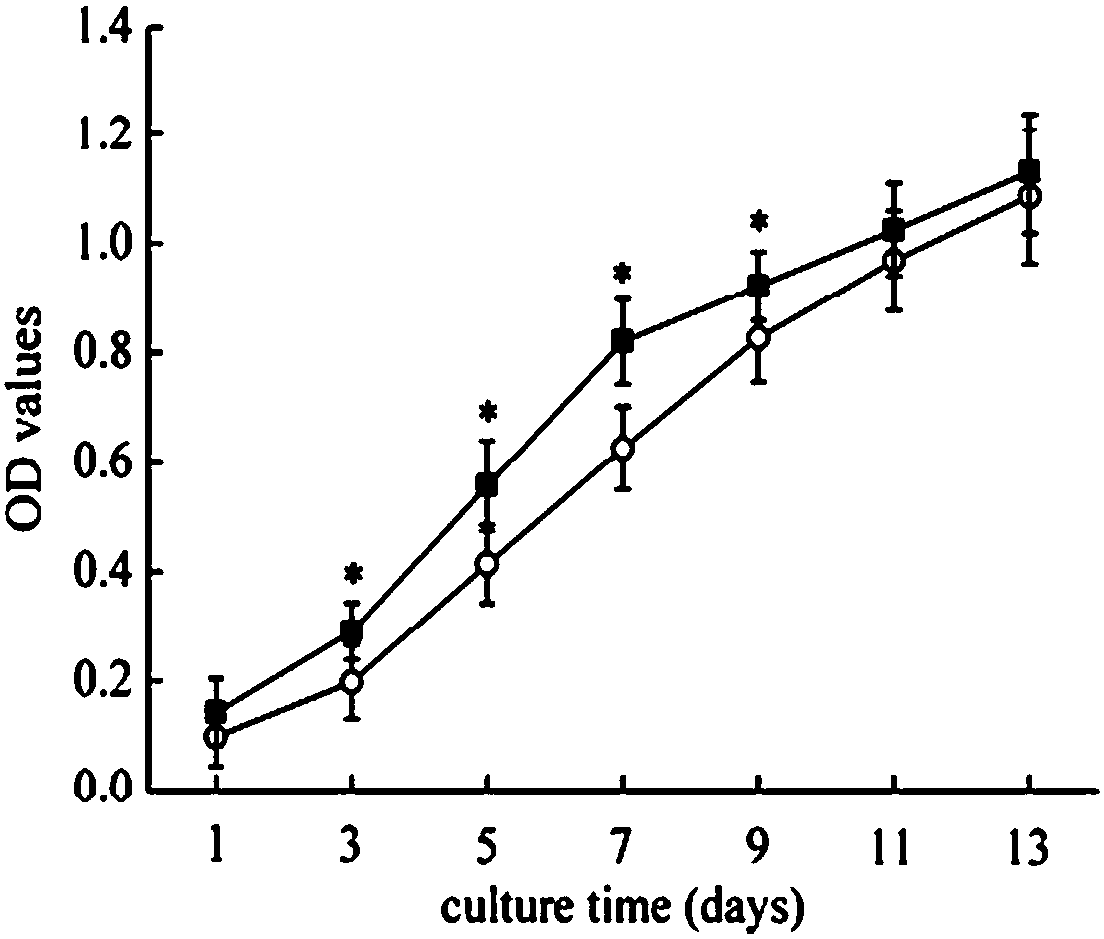
Exosome-compounded collagen biological scaffold with directional releasing function as well as preparation method and application thereof
A collagen biology and exosome technology, applied in the field of medical biomaterials, can solve the problems of difficulty in forming a target, and MSC-Exo cannot be administered in a concentrated area, so as to achieve effective retention of activity and improve targeted repair and therapeutic effects. , the preparation method is simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention will be described in further detail below in conjunction with the accompanying drawings and embodiments.
[0033] 1. Main reagents and instruments
[0034] Pepsin, Chloroform, Glacial Acetic Acid, NaHCO 3 , NaCl, high-speed centrifuge, freeze dryer, deep-low temperature refrigerator, meat grinder, dialysis membrane, liquid nitrogen planetary ball mill.
[0035] 2. Method
[0036] 2.1 Collagen Preparation
[0037] (1) Pretreatment: take fresh bovine (pig, sheep or other animal) Achilles tendon, bovine (pig, sheep or other animal) skin, bovine (pig, sheep or other animal) articular cartilage and other biological materials (for example, take skin), wash and remove subcutaneous fat and dermis, remove fascia, remove hair, and chop finely.
[0038] (2) Degreasing: The shredded biological material is washed with double distilled water, minced in a meat grinder, soaked in a mixed solvent of chloroform-methanol (for example, volume ratio 1:1) at 2-6°C (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com