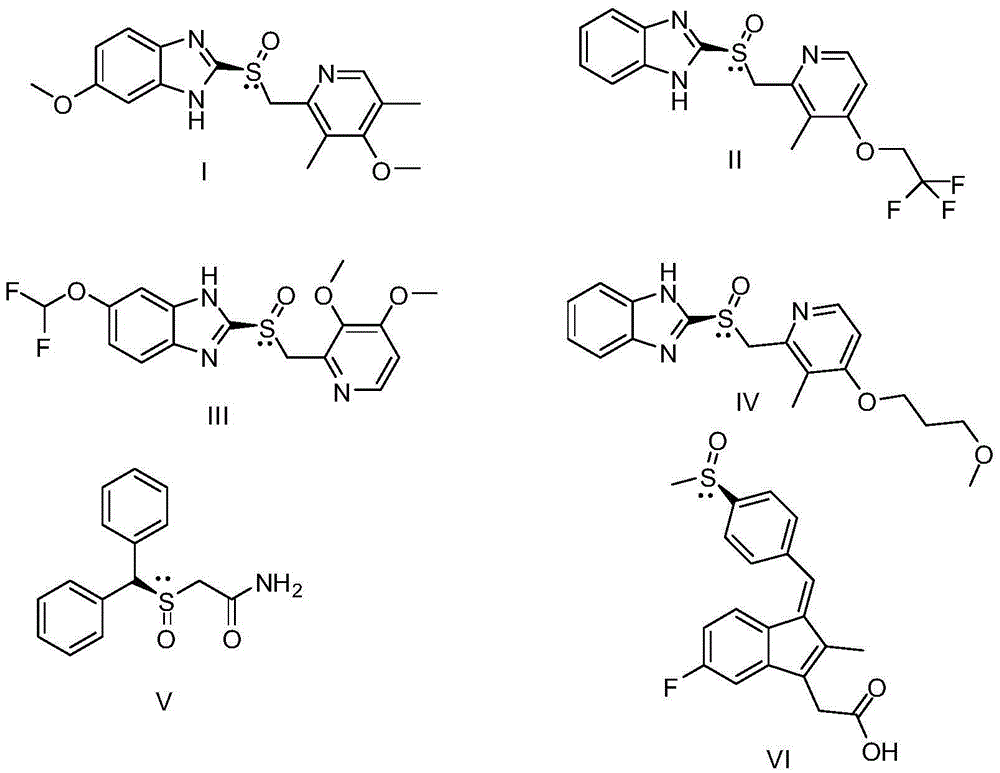
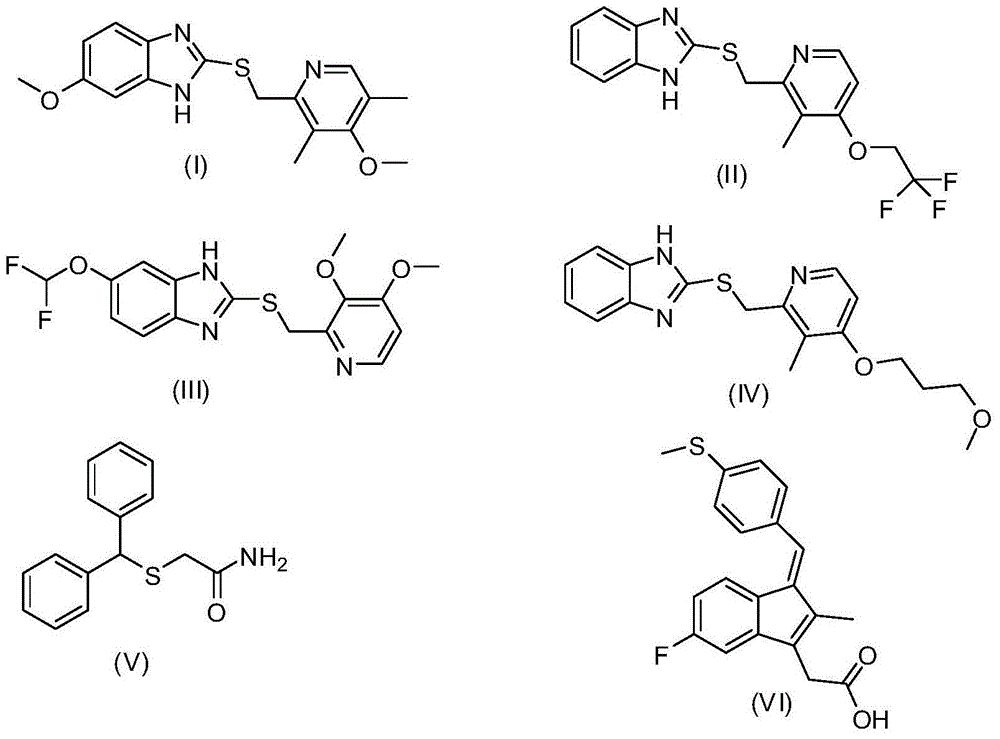
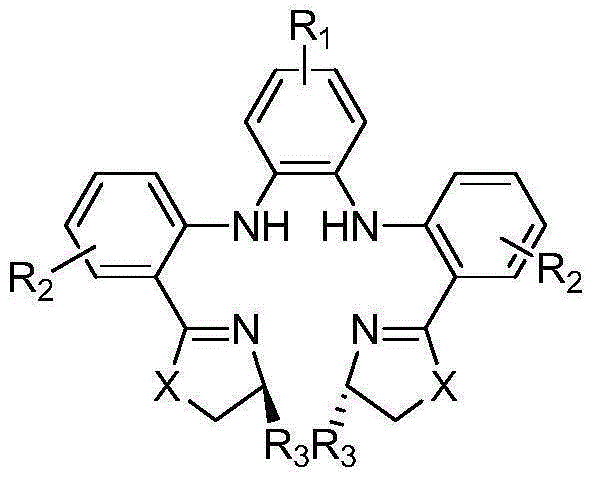
Preparation method of chiral sulfoxide medicament though catalysis of asymmetric oxidation of sulfides compound
A sulfoxide-based, asymmetric technology, applied in the field of catalytic asymmetric oxidation of prochiral sulfides to prepare chiral sulfoxide drugs, can solve the problem of increasing the complexity of the reaction system and operating costs, the use of large amounts of chiral ligands, Limit the industrial production of chiral sulfoxide drugs and other problems, and achieve the effects of high conversion rate, mild reaction conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of a chiral tetradentate nitrogen ligand
[0032]
[0033]Add 22.5 mg (0.1 mmol) of palladium acetate and 72 mg (0.3 mmol) of tri-tert-butylphosphine into 50 mL of toluene solution, and stir for 10 min. 2.36 g (10 mmol) of o-dibromobenzene, 3.63 g (24 mmol) of methyl 2-aminobenzoate and 10.1 g (31 mmol) of cesium carbonate were successively added. After the reaction solution was heated to reflux for 24 hours, it was cooled to 25°C, and 50 mL of saturated ammonium chloride solution was added. 200 mL of dichloromethane was added, the organic phase was separated, and the aqueous phase was extracted twice with 60 mL of dichloromethane each time. The organic phases were combined, dried, concentrated, and 1.47 g of compound 1 (yield 39%) was obtained by column chromatography (ethyl acetate / petroleum ether=1:50). 1 HNMR (400MHz, CDCl 3 )δ9.21(2H,s),7.89(2H,d,J=7.4),7.42(2H,s),7.25(3H,s),7.11(2H,d,J=2.8),7.04(2H, d,J=8.1),6.70(2H,s),3.80(6H,s). 13 CNMR (101MHz...
Embodiment 2
[0038] Embodiment 2 (S) - the synthesis of omeprazole
[0039]
[0040] At 25°C, add Mn(OTf) to 3.0 mL of dichloromethane 2 (1.5mg, 0.0042mmol) and L2 (2.0mg, 0.0042mmol) were stirred for 3h. Then 0.42 mmol thioether and 2.1 mmol glacial acetic acid and 30% hydrogen peroxide (0.92 mmol) were added. The reaction mixture was cooled to 0 °C and stirred at 0 °C for 1 h. The organic phase was separated, dried, analyzed by high performance liquid chromatography to obtain the ee value, and the product was obtained by column chromatography, and the yield was calculated.
[0041] Using 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfanyl]-1H-benzimidazole as a model substrate versus reaction conditions optimize. The results are shown in the table below.
[0042]
[0043]
[0044] [a] Separation yield. [b] Chiral HPLC determination.
[0045] It can be seen from the table that the solvent is dichloromethane, the molar ratio of hydrogen peroxide to the substrate ...
Embodiment 3
[0047] The preparation of embodiment 3S-lansoprazole
[0048] Experimental procedure is identical with embodiment 2. 89% yield, 98% ee value.
[0049] 1 HNMR (300MHz, DMSO-d 6 ):δ13.6(brs,1H),8.28(d,J=5.6Hz,1H),7.65(brs,2H),7.30(m,2H),7.09(d,J=5.6Hz,1H),4.90 (q,J=8.7Hz,2H),4.83and4.75(AB-system,J=13.7Hz,2H),2.17(s,3H).[a] D 25 =-199.7(c1.0,acetone).Ee value is determined by chiral high performance liquid chromatography (chromatographic column: Kromasil KR100-5CHI-TBB mobile phase: n-hexane / isopropanol / acetic acid / triethylamine (volume ratio )=90:10:0.1:0.2, flow rate: 1.5mL / min, wavelength 284nm, retention time=8.3min, 10.4min)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com