Method for preparing (S)-pantoprazole in high-enantioselectivity way
A technology of pantoprazole and pantoprazole sulfide, which is applied in the field of highly enantioselective preparation of pantoprazole, can solve the problems of cumbersome and troublesome, insufficient chiral purity, cumbersome separation operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
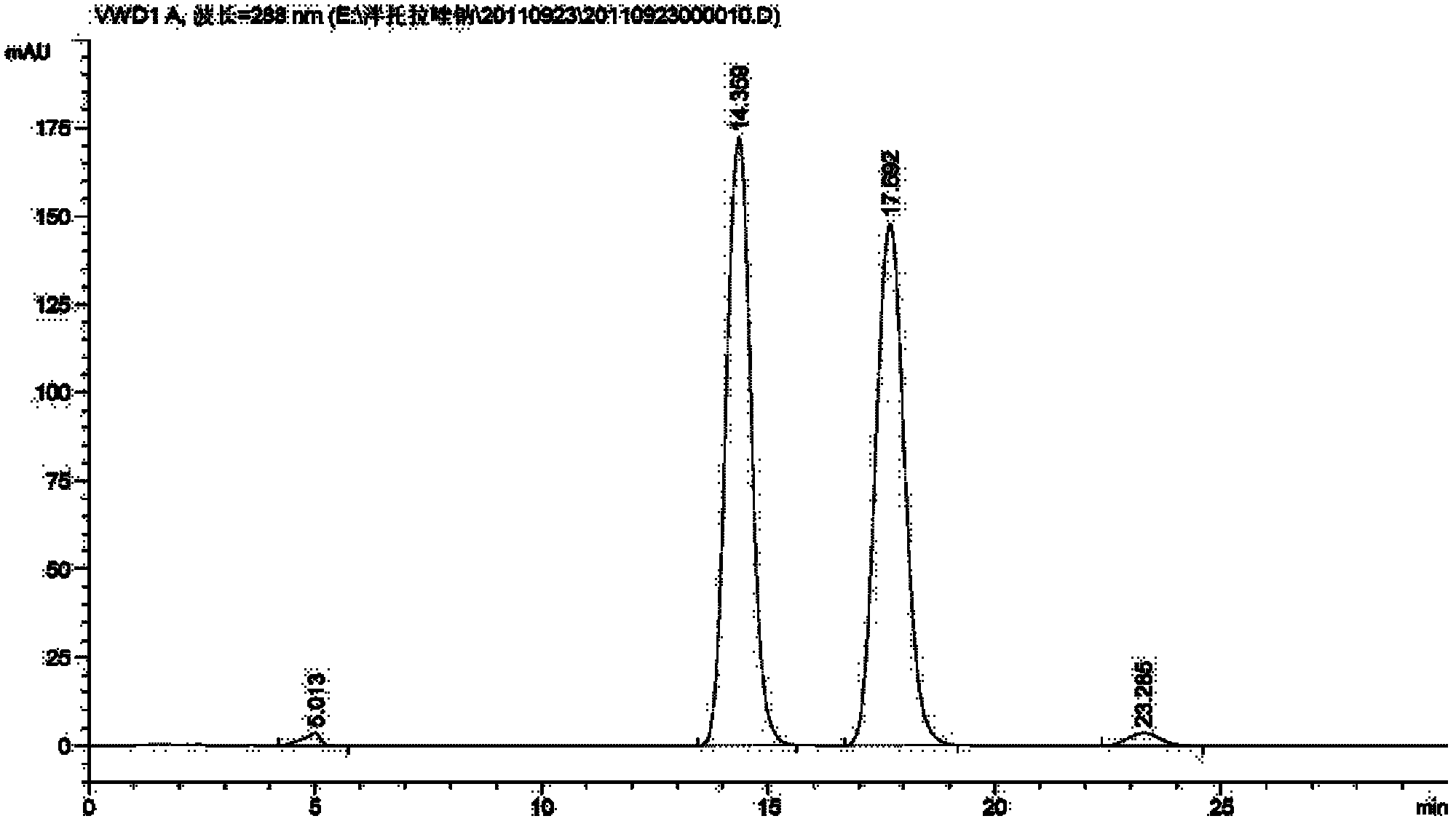
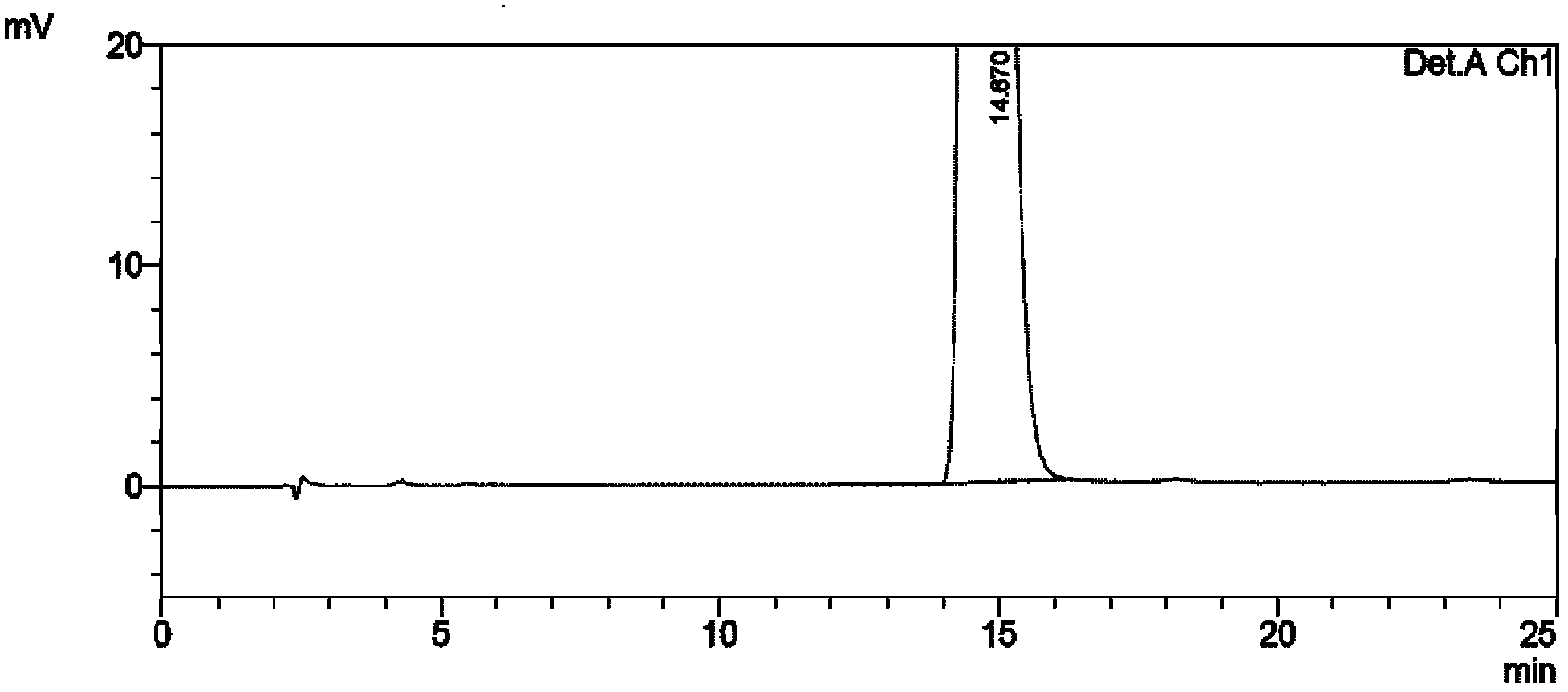
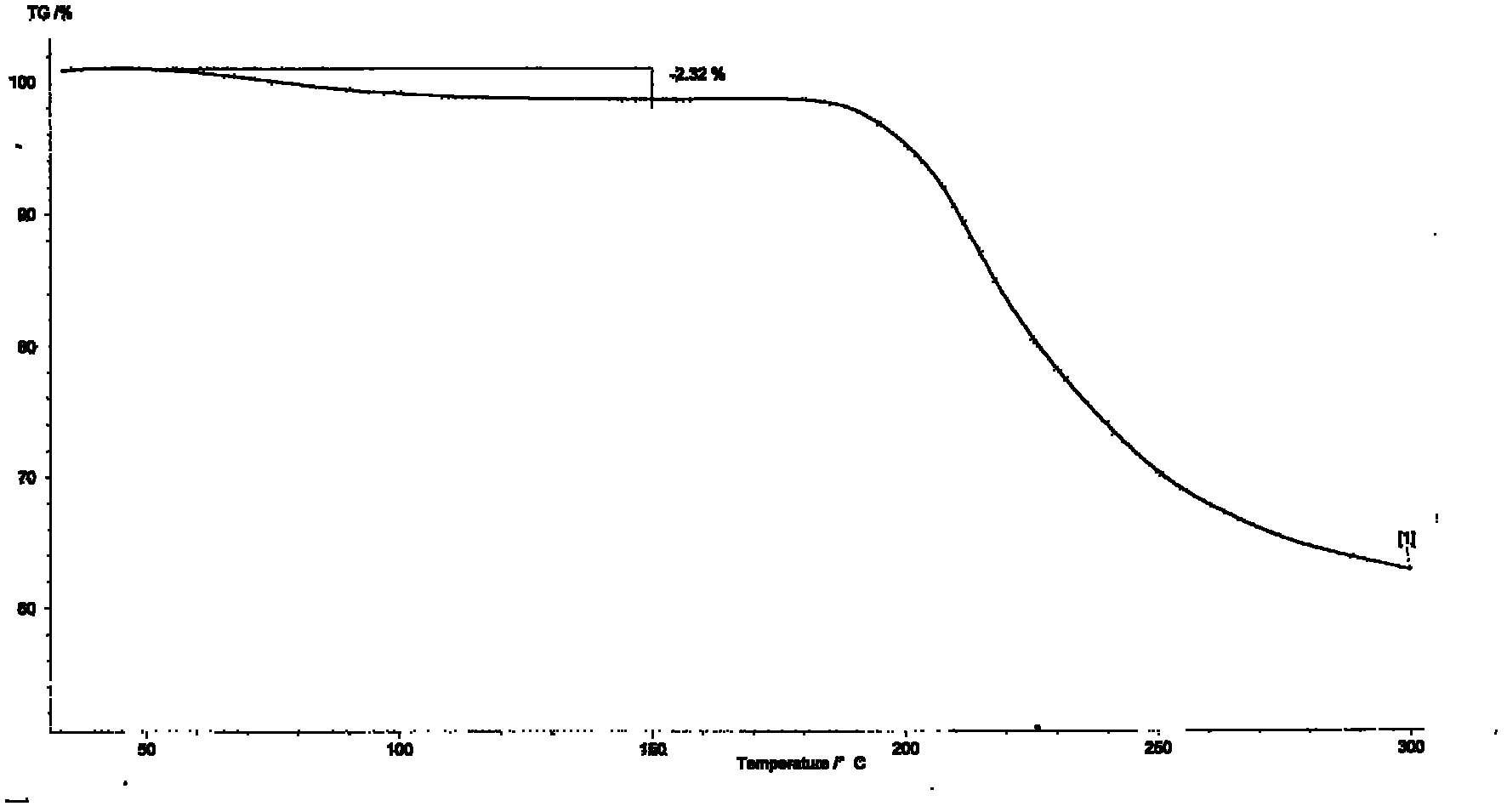
[0033] Dissolve (1R,2S)-1-amino-2-indanol 0.01mol in 20ml acetonitrile, add Ti(OiPr) under stirring 4(0.005mol), cooled to 15 ° C, stirred for 15 minutes, then added pantoprazole sulfide (0.01mol), then slowly added dropwise cumene hydroperoxide 0.0232mol, continued to react for 3 hours, added to the reaction system 20ml of toluene, stirred for 10min, then added 12.5% ammonia water (50ml×3) and stirred for 20min each, separated the layers, combined the ammonia water layer, then adjusted the pH to 7.5-8 with glacial acetic acid in an ice-water bath, and then added ethyl acetate (50ml × 3) extraction, combined organic layers, then distilled off ethyl acetate, the obtained (S)-pantoprazole free body was dissolved with 3ml of ethyl acetate, then added dropwise with 3ml petroleum ether and stirred, filtered, below 40°C After vacuum drying, 2.40 g of (S)-pantoprazole was obtained after purification. Yield: 62.66%; Ee (%): 100%; see relevant graph Figure 1 to Figure 5 .
[0034...
Embodiment 2
[0036] (1R,2S)-1-Amino-2-indanol 0.005mol was dissolved in 20ml of dichloromethane, and Ti(OiPr) was added under stirring 4 (0.005mol), cooled to -12 ℃, stirred for 15 minutes, then added pantoprazole sulfide (0.01mol), then slowly added dropwise cumene hydroperoxide 0.01mol, continued to react for 20 hours, and added to the reaction system Add 20ml of toluene, stir for 10min, then add 12.5% ammonia water (50ml×3) and stir for 20min each, separate the layers, combine the ammonia water layer, then adjust the pH to 7.5-8 with glacial acetic acid in an ice-water bath, and then use ethyl acetate ( 50ml×3) extraction, combined organic layers, then evaporated ethyl acetate, the obtained (S)-pantoprazole free body was dissolved with 3ml of ethyl acetate, then added dropwise with 3ml of petroleum ether and stirred, filtered, 40°C Following vacuum drying, 1.43 g of (S)-pantoprazole was obtained after purification. Yield: 37.33%; Ee(%): 45.86%.
Embodiment 3
[0038] (1R,2S)-1-Amino-2-indanol 0.0067mol was dissolved in 20ml of dichloromethane, and Ti(OiPr) was added under stirring 4 (0.005mol), cooled to 0 ℃, stirred for 15 minutes, then added pantoprazole sulfide (0.01mol), then slowly added dropwise cumene hydroperoxide 0.015mol, continued to react for 11 hours, added to the reaction system 20ml of toluene, stirred for 10min, then added 12.5% ammonia water (50ml×3) and stirred for 20min each, separated the layers, combined the ammonia water layer, then adjusted the pH to 7.5-8 with glacial acetic acid in an ice-water bath, and then added ethyl acetate (50ml × 3) extraction, combined organic layers, then distilled off ethyl acetate, the obtained (S)-pantoprazole free body was dissolved with 3ml of ethyl acetate, then added dropwise with 3ml petroleum ether and stirred, filtered, below 40°C After vacuum drying, 1.54 g of (S)-pantoprazole was obtained after purification. Yield: 40.21%; Ee(%): 53.27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com