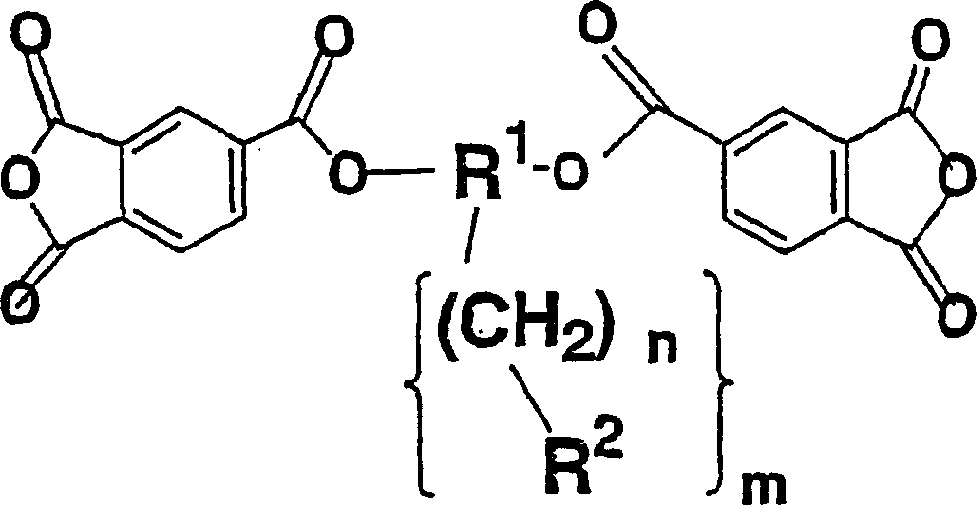
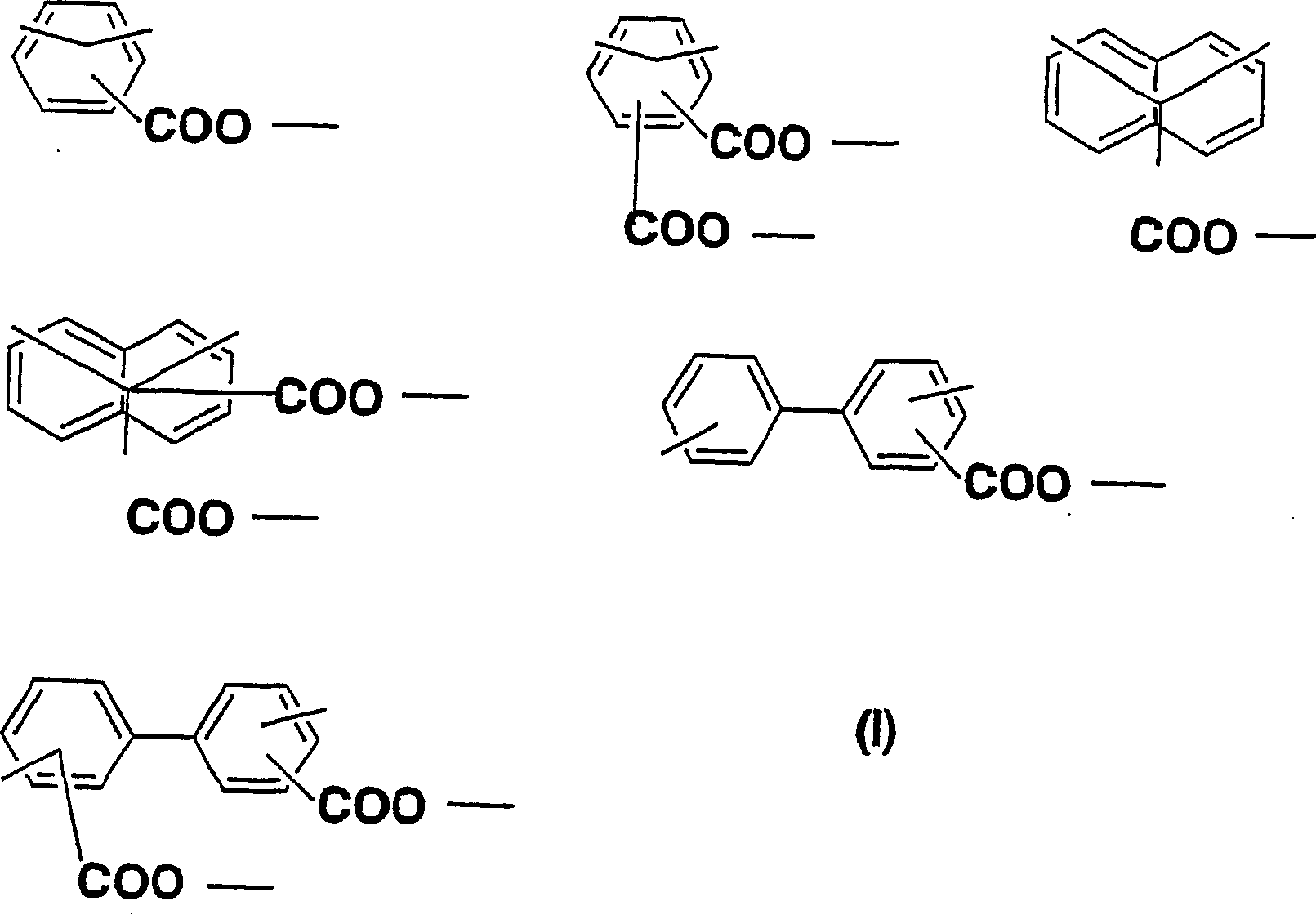
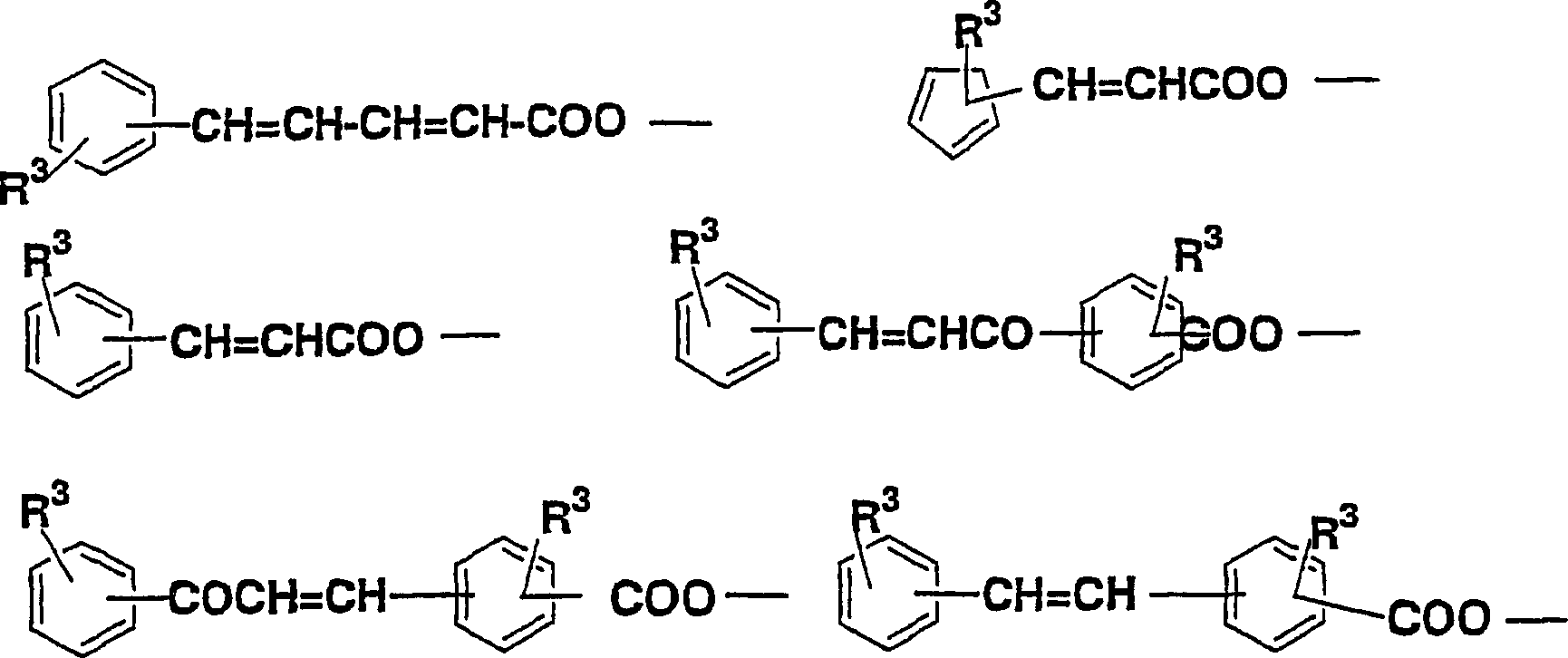
Diamine, acid dianhydride, polyimide composition having reactive group obtained therefrom, and processes for producing these
A technology of reactive groups and acid dianhydrides, applied in the field of new polyimide compositions, can solve problems such as unknown diamines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0306] (1-1) Synthesis of 12-bromododecane-1-(4-fluorocinnamate)
[0307] Put 4.6g (25 mmol) of 4-fluorocinnamic acid chloride and 50 ml of methyl ethyl ketone into the reactor, then slowly add 5.0 g (18.9 mmol) of 12-bromododecanol, three A solution of 3.0 g (30 mmol) of ethylamine dissolved in 50 ml of methyl ethyl ketone was stirred under reflux under a nitrogen stream. The generated onium salt was filtered off, concentrated to dryness, and then recrystallized from methanol to obtain 7.4 g (17.9 mmol) of 12-bromododecane-1-(4-fluorocinnamate). Yield 95%.
[0308] 1 H-NMR (solvent CDCl 3 ): δ7.85~6.93(m, Ph-H, 4H), 6.50(s, CH=CH, 1H), 6.25(s, CH=CH, 1H), 4.20(t, CH, 2H), 3.40( t, CH, 2H), 1.90~0.9 (m, CH, 20H)
[0309] (1-2) Synthesis of 12-(4-fluorocinnamic acid)-dodecane-1-(2,5-dihydroxybenzoate)
[0310] 7.33 g (17.5 mmol) of 12-bromododecane-1-(4-fluorocinnamate), 5.72 g (20 mmol) of cesium salt of 2,5-dihydroxybenzoic acid, dimethylformamide 50 ml was used as a r...
Embodiment 2~6
[0317] The following examples 2 to 6 are synthesis examples of diamines with reactive groups of the present invention
Embodiment 2
[0319] (2-1) Synthesis of 1-dodecanol-12-(4-fluorocinnamate)
[0320] Put 7.23g (25 mmol) of cesium salt of cinnamic acid, 5.0 g (18.9 mmol) of 12-bromododecanol, and 50 ml of dimethylformamide (hereinafter referred to as DMF) into the reactor, and carry out the process at 100 ° C. Heating was continued with stirring for 2 hours. (The reaction was carried out under nitrogen flow). After filtering the reaction solution, the filtrate was poured into water, and the precipitated white solid was filtered to obtain 6.26 g (18.3 mmol) of 1-dodecanol-12-(4-fluorocinnamate). (yield 97%)
[0321] 1 H-NMR (solvent CDCl 3 ): δ7.85~6.93(m, Ph-H, 4H), 6.50(s, CH=CH, 1H), 6.26(s, CH=CH, 1H), 4.20(t, CH, 4H), 3.76( t, CH, 4H), 1.90~0.8(m, CH, 20H), 1.8(s, OH, 1H)
[0322] (2-2) Synthesis of 12-(4-fluorocinnamic acid)-dodecane-1-(3,5-dinitrobenzoate)
[0323] Put 1-dodecanol-12-(4-fluorocinnamate) 6.15g (18 mmol), triethylamine 2.5g (25 mmol), methyl ethyl ketone 60ml into the reactor, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com