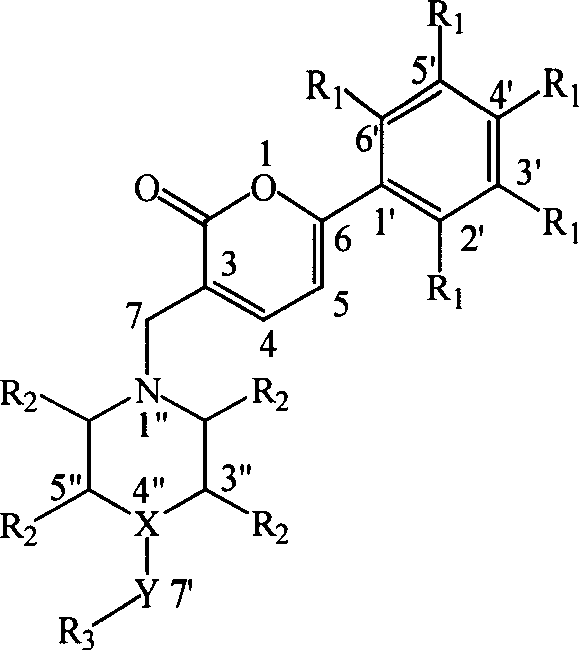
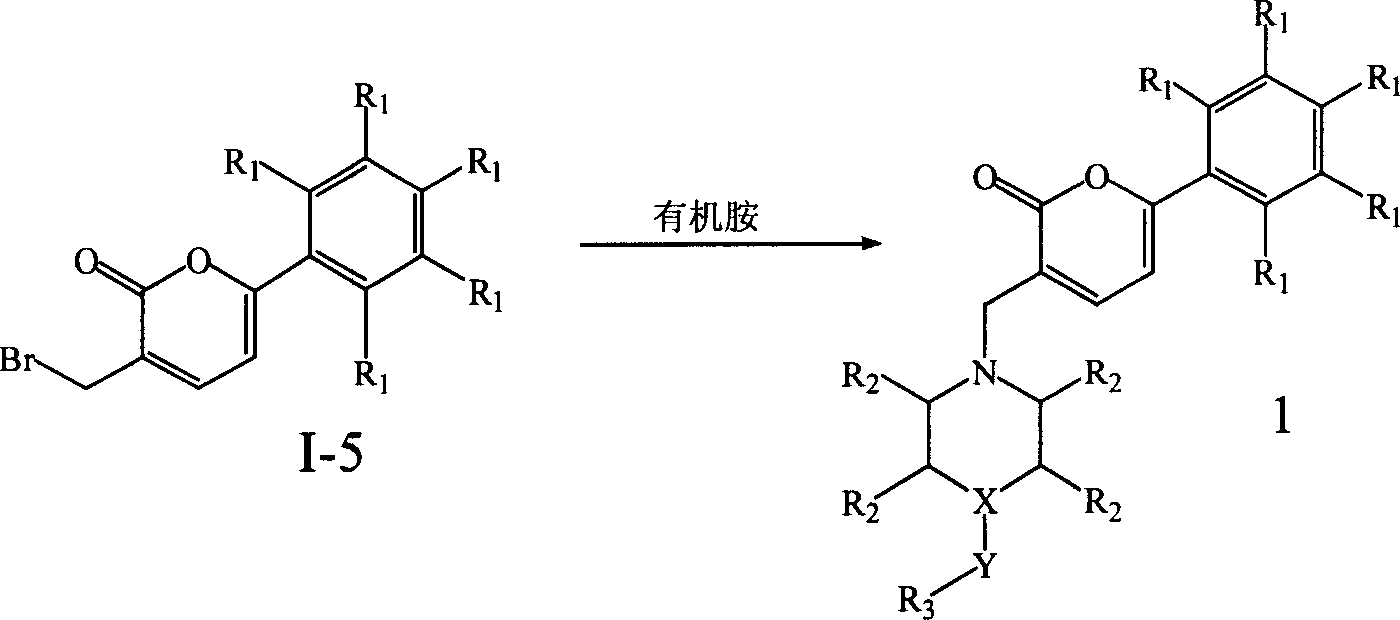
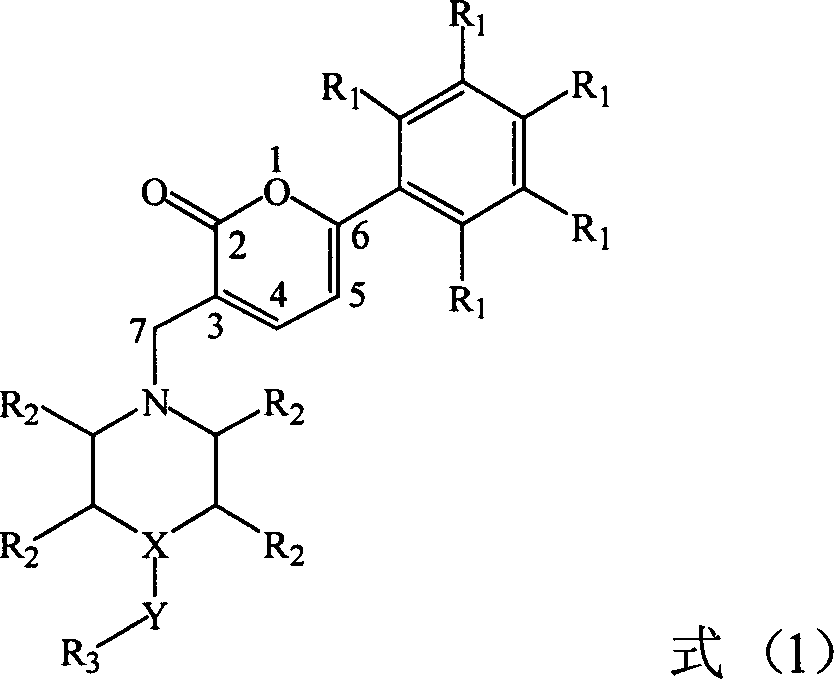
Preparation and application of a category of 6 - aryl - 3 - cycroamido methyl pyrone derviation
A kind of cyclic aminomethylpyrone and base methyl technology is applied in the field of 6-aryl 3-cyclic aminomethylpyrone derivatives and their preparation, which can solve the problem of large toxic and side reactions for patients, affecting the dosage and Scope of use and sales, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0063] Preparation Example 1: Preparation of Compound I-0 (ethyl 2-methylacetoacetate):
[0064]
[0065] In 300 milliliters of ethanol, add 13.8 grams of metal sodium (600 mmol), after completely dissolving, add dropwise 80 ml (600 mmol) of ethyl acetoacetate, heat up and reflux for 1 hour, dropwise add 50 ml of iodomethane (660 mmol), reflux for two hours, cool Return to room temperature, remove ethanol by rotary evaporation, add water and extract with ether (5×50ml), combine organic phases, dry over anhydrous sodium sulfate, filter with suction, remove ether by rotary evaporation, distill under reduced pressure, collect 78-80°C (13mmHg) fraction , to obtain 87.63 grams of colorless transparent liquid, the separation yield Y=50.71%.
preparation example 2
[0066] Preparation Example 2: Preparation of Compound I-1 [with (2-methyl-3-oxo-5-hydroxyl-5-(3',4',5'-trimethoxyphenyl)-pentanoic acid ethyl ester) For example]:
[0067]
[0068]Under ice-salt bath cooling conditions, 5.78 g (37.5 mmol) of ethyl 2-methylacetoacetate was added dropwise to 2.3 g of sodium hydride (95.8 mmol) in tetrahydrofuran suspension (140 ml), and then 2.5 M 28ml (70.2mmol) of n-hexane solution of butyllithium, after ten minutes, dropwise add 5.88 grams (30mmol) of tetrahydrofuran solution (10ml) of 3,4,5-trimethoxybenzaldehyde, continue to react for 15 minutes, add to the reaction system Add saturated aqueous ammonium chloride solution (30ml), extract with diethyl ether (3×50ml), combine organic phases, wash with saturated brine (3×20ml), dry over anhydrous sodium sulfate, suction filter, silica gel column chromatography (petroleum ether / ethyl acetate: 2 / 1) 7.572 g of yellow oily liquid was obtained, and the separation yield Y=72.11%.
[0069] Rf (e...
preparation example 3
[0070] Preparation Example 3: Preparation of Compound I-2 [taking (2-methyl-3-hydroxyl-5-hydroxyl-5-(3',4',5'-trimethoxy)-ethyl valerate) as an example 】:
[0071]
[0072] In an ice-water bath, 3.06 g (9 mmol) of intermediate I-1 was dissolved in tetrahydrofuran (67.5 ml) and methanol (27 ml), and 1.04 g (27 mmol) of sodium borohydride was slowly added to react for 30 minutes. Add 30 ml of saturated aqueous ammonium chloride solution, extract with ether (3×40 ml), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and remove ether by rotary evaporation to obtain 3.22 g of crude product, which is directly carried out to the next reaction.
[0073] Rf (ethyl acetate / petroleum ether: 1 / 1): 0.35.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com