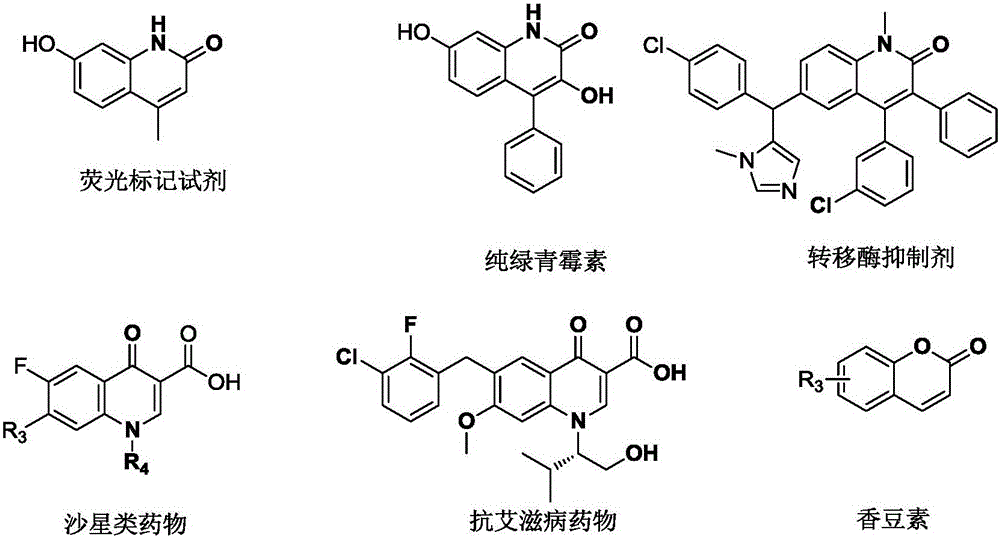
Synthesis method of difluoroalkyl substituted pyridone or pyrone
The technology of a difluoroalkyl group and a synthesis method is applied in the synthesis field of pyridone or pyranone, and achieves the effects that raw materials and reagents are simple and easy to obtain, the reaction is green and concise, and the dosage is small.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
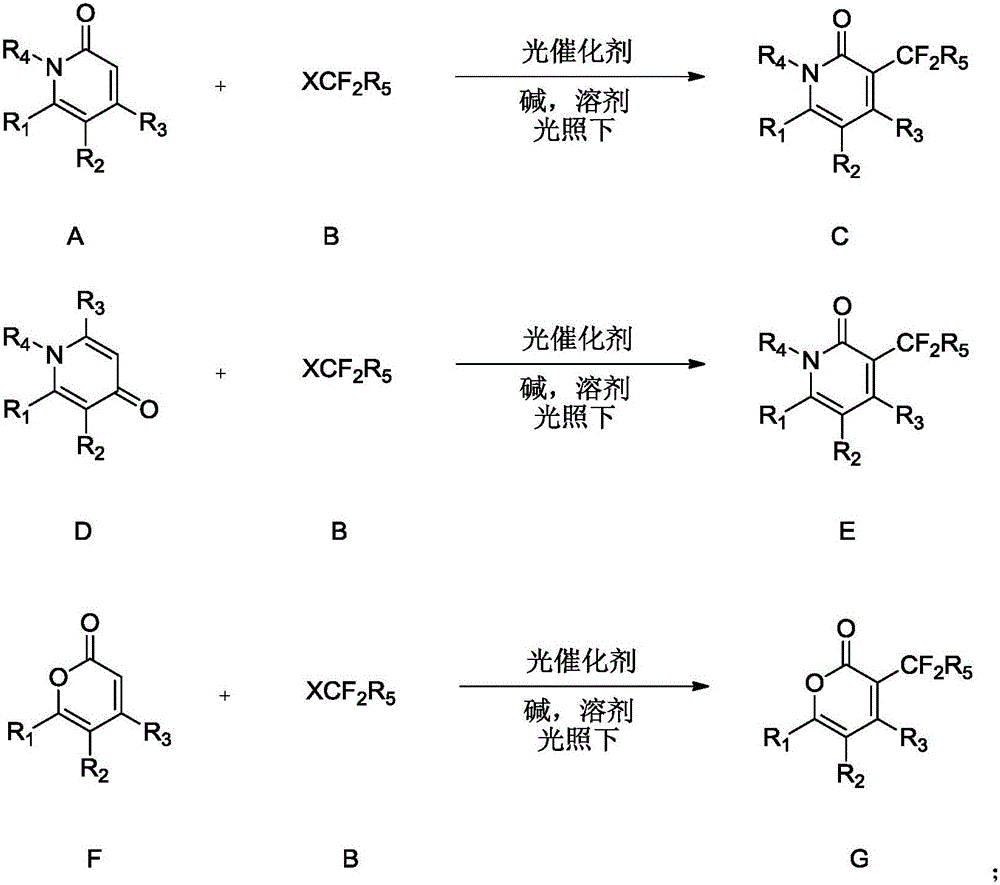
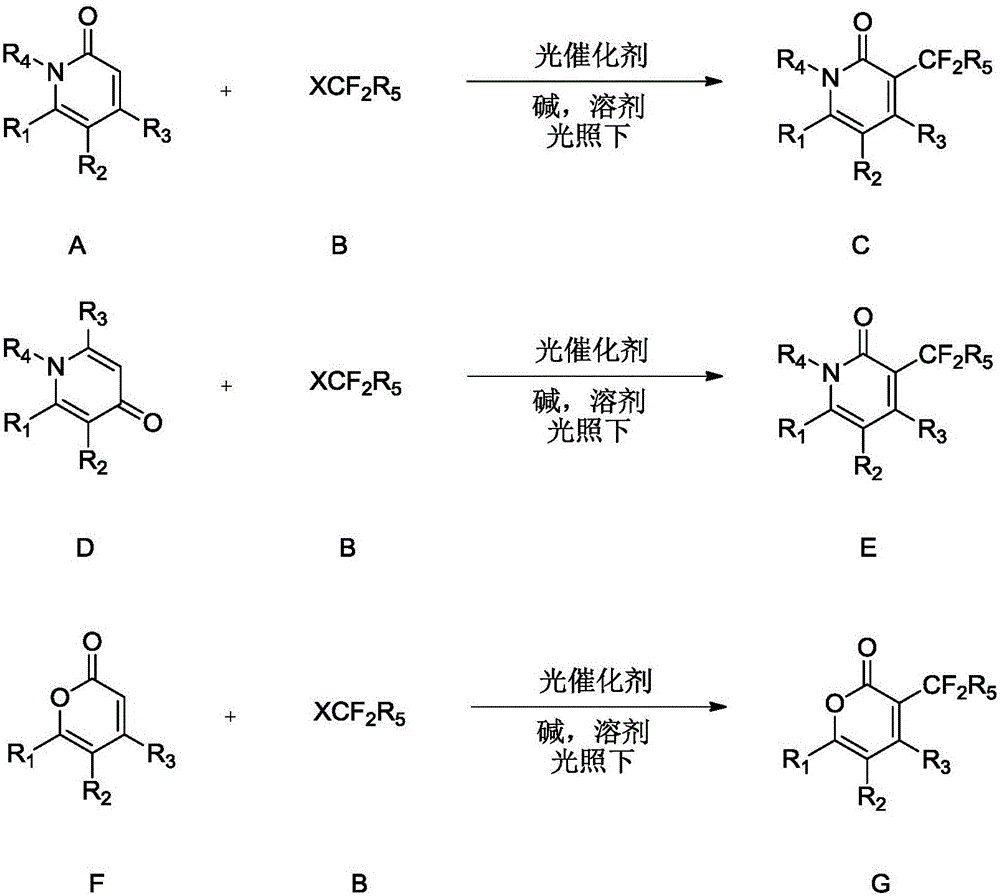
[0033] The invention provides a method for synthesizing difluoroalkyl substituted pyridone or pyrone. Preferably, the method includes the steps of: in an inert solvent, under the irradiation of blue light or green light, using a complex containing iridium and ruthenium as a photocatalyst, the formula pyridone / pyrone (ie A, D or F) react with a compound of formula B) for a period of time (such as 1-36 hours) to form a difluoroalkyl substituted pyridone or pyrone (compound C, E or G);
[0034]
[0035] In various forms, R 1 , R 2 , R 3 , R 4 , R 5 , X is defined as mentioned above.
[0036] More preferably, said formula A, E, F compound is selected from the compound of following group:
[0037]
[0038] Wherein, the compound of formula B is preferably a compound selected from the following group:
[0039] ClCF 2 R 5 , BrCF 2 R 5 、ICF 2 R 5 .
[0040] In various forms, R 4 as above.
[0041] More preferably, the compound of formula B is a compound selected f...
Embodiment 1
[0055]
[0056] Into a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy) 3 , K 2 HPO 4 (0.8mmol), compound A-1 (0.4mmol, 1 equivalent), after nitrogen replacement three times, add 3mL dimethylsulfoxide (DMSO), inject 100μL (0.80mmol) compound B-1, stir under blue light irradiation for 24 hours , to obtain compound C-1. The yield was 84%.
[0057] 1 HNMR (400MHz, CDCl 3 )δ8.11(s, 1H), 7.64(t, J=7.2Hz, 2H), 7.37(d, J=8.8Hz, 1H), 7.29(t, J=7.4Hz, 1H), 4.37(q, J=7.2Hz, 2H), 3.67(s, 3H), 1.33(t, J=7.2Hz, 3H). 19 FNMR (376MHz, CDCl 3 )δ-109.5(s,2F). 13 C-NMR (100MHz, CDCl 3 )δ163.1(t, J=32.6Hz), 159.2(t, J=4.4Hz), 140.2, 137.3(t, J=7.1Hz), 132.3, 130.0, 124.7(t, J=24.1Hz), 122.8 , 118.7, 114.2, 111.2 (t, J = 247.6 Hz), 62.9, 29.2, 13.8.
Embodiment 2
[0059]
[0060] Into a 25mL reaction tube, add 1.3mg (0.5mol%) Ir(PPy) 3 , K 2 HPO 4 (0.8mmol), compound A-1 (0.4mmol, 1 equivalent), after nitrogen replacement three times, add 3mL dimethylsulfoxide (DMSO), inject 100μL (0.80mmol) compound B-1, and stir for 24 hours under green light irradiation Finally, compound C-1 was obtained. The yield was 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com