Slow-release small pill prparation containing huperzine A and method for making same
A technology of Huperzine A and sustained-release pellets, applied in the field of pharmaceutical preparations, can solve problems such as unfavorable treatment of patients, inconvenient dosage forms of Huperzine A, and achieve the effects of reducing toxic side effects, improving bioavailability, and completely releasing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
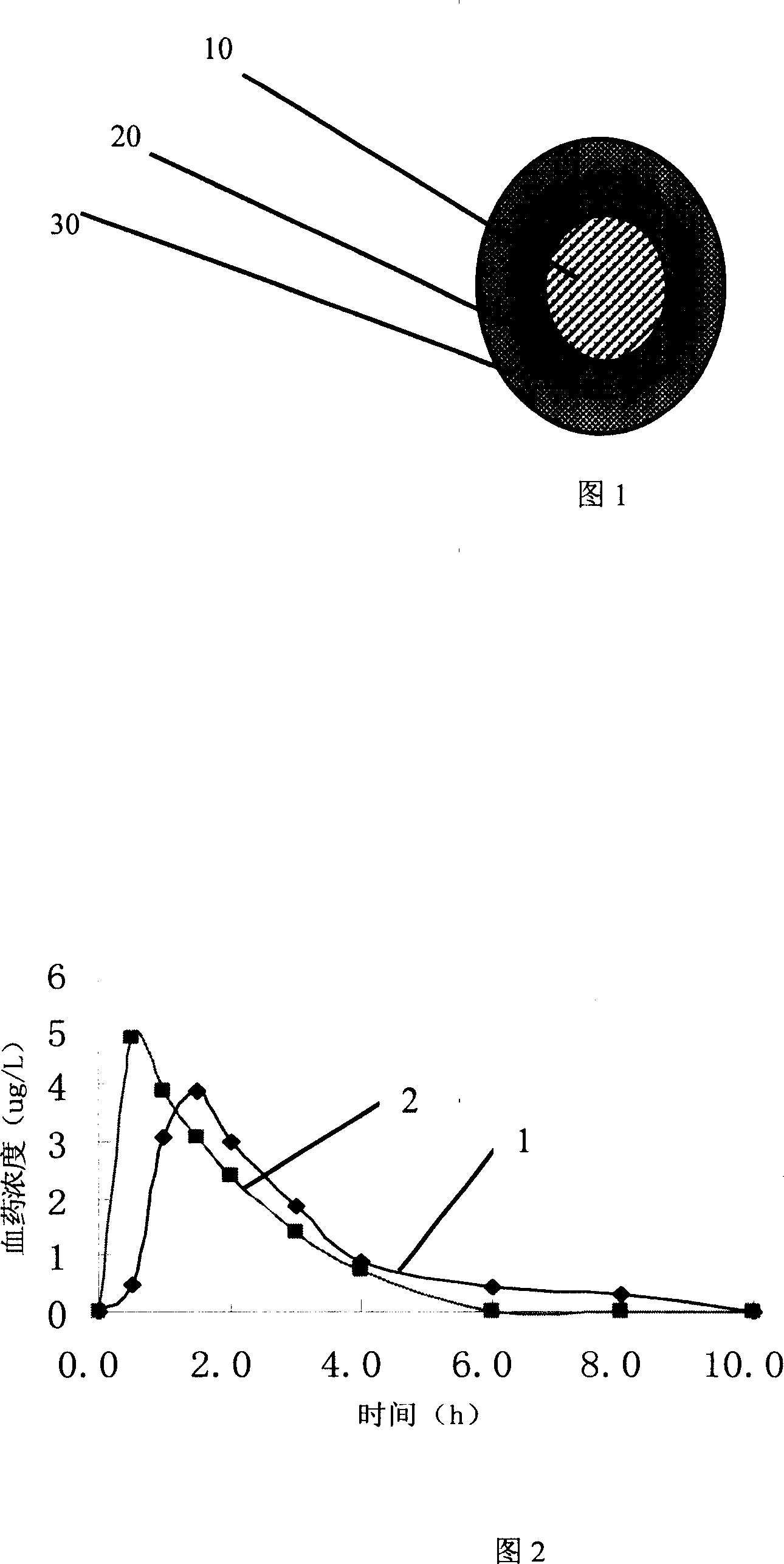
Image
Examples
Embodiment 1
[0039] Embodiment 1 0.044% (weight) huperzine A sustained-release pellet preparation
[0040] All of the various substances described below are expressed in grams of dry matter for a final amount of 5000 g sustained release pellet formulation.
[0041] composition
[0042] Neutral ball core 4432.62
[0043] Active layer:
[0044] Huperzine A 2.22
[0045] Polyacrylic resin RS 4.43
[0046] Slow release layer:
[0047] Ethyl cellulose 443.26
[0048] Dibutyl sebacate 106.38
[0050] Steps:
[0051] A. Dissolve huperzine A, or the acid salt of pharmaceutically acceptable huperzine A and binding agent—polyacrylic acid resin RS in the aqueous ethanol solution, in the described aqueous ethanol solution, the The mass percentage concentration of ethanol is 50~95%;
[0052] B. Prepare the neutral ball core, spray the solution prepared above onto the neutral ball core and dry it, forming an active layer on the outside of the neutral ball core;
[0...
Embodiment 2
[0055] Embodiment 2 0.008% (weight) huperzine A sustained-release pellet preparation
[0056] All of the various substances described below are expressed in g of dry matter for a final amount of 5000 g sustained release pellet formulation (preparation as above).
[0057] composition
[0058] Neutral ball core 4440.0
[0059] Active layer:
[0060] Huperzine A 0.4
[0061] Polyvinylpyrrolidone 2.0
[0062] Slow release layer:
[0063] Polyacrylic resin RS type 491.0
[0064] Triethyl citrate 49.1
[0065] Talc 17.5
Embodiment 3
[0066] Embodiment 3 0.015% (weight) huperzine A sustained-release pellet preparation
[0067] All of the various substances described below are expressed in g of dry matter for a final amount of 5000 g sustained release pellet formulation (preparation as above).
[0068] composition
[0069] Neutral ball core 4500.0
[0070] Active layer:
[0071] Huperzine A 0.75
[0072] Methylcellulose 3.0
[0073] Slow release layer:
[0074] Ethyl cellulose 360.3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com