Huperzine-A framework particles, orally disintegrating tablets and preparation methods thereof
A technology for huperzine A and orally disintegrating tablets, which is applied to pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficulty swallowing, poor patient compliance, and uneven content , to achieve the effect of stable process, good reproducibility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
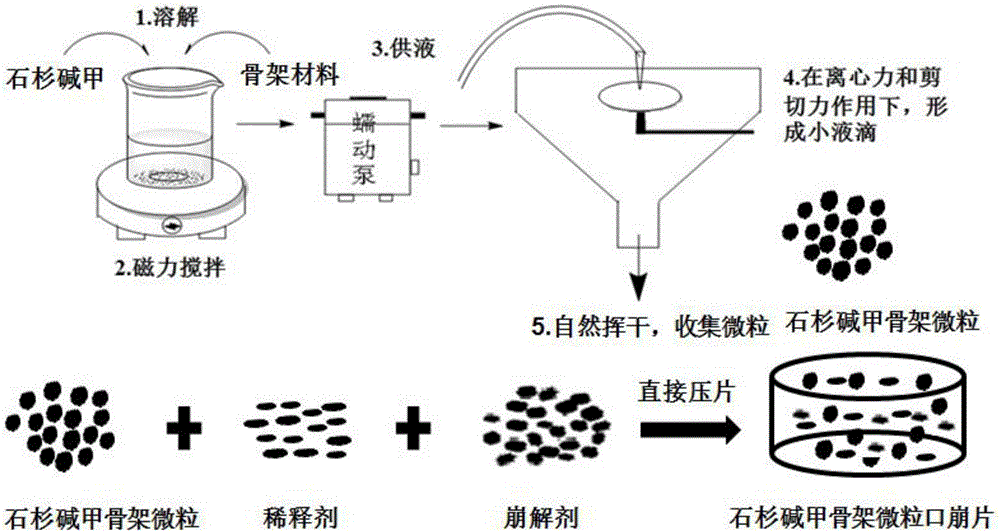
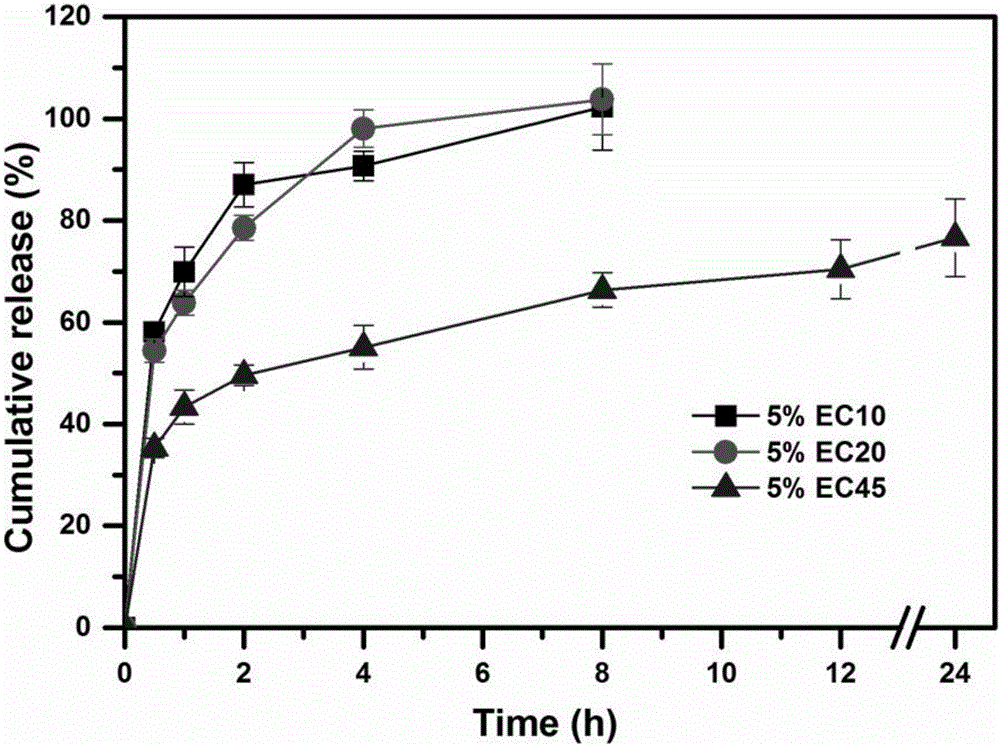
[0052] The theoretical drug loading capacity of the fixed huperzine A matrix particles is 0.2% (w / v). Take by weighing an appropriate amount of huperzine A, be dissolved in 200ml 80% v / v ethanol solution, obtain drug solution, slowly add a certain amount of skeleton material (ethyl cellulose EC (EC7 / EC10 / EC20 / EC45), acrylic resin Eudragit RSPO or vinyl acetate-povidone blend Kollidon SR), to form a drug-containing polymer solution with a concentration of 5% to 20% (w / v) of the skeleton material . The drug-containing polymer solution is fed into the UPPS at an average speed of 7.8ml / min through a peristaltic pump, and is sheared and atomized under the action of a high-speed rotating disc (9000rpm) to form droplets. The solvent in the droplets is in the airflow field The huperzine A skeleton microparticles are obtained by evaporating in medium, solidifying the microdroplets, and finally collecting the huperzine A skeleton microparticles. The yield and formability of huperzine...
Embodiment 2
[0057] Weigh 20mg of huperzine A, dissolve in 200ml 80% v / v ethanol solution to obtain a drug solution, slowly add 10g of skeleton materials EC10, EC20 and EC45 to the drug solution under the action of magnetic stirring, until the swelling of EC is completely formed Uniform drug-containing polymer solution. The drug-containing polymer solution is fed into the UPPS at an average speed of 7.8ml / min through a peristaltic pump, and micro-droplets are formed under the action of a 9000rpm high-speed rotating disc. Huperzine A skeleton microparticles were obtained. The prepared huperzine A matrix particles were stored in a desiccator for subsequent research.
[0058] The assay method of drug content in the huperzine A skeleton particle (being the assay method of drug encapsulation efficiency) is: accurately weigh 10~10.5mg huperzine A skeleton particle and place in 5ml volumetric flask, add 80% methanol ultrasonic to After the particle skeleton is completely dissolved, adjust to vo...
Embodiment 3
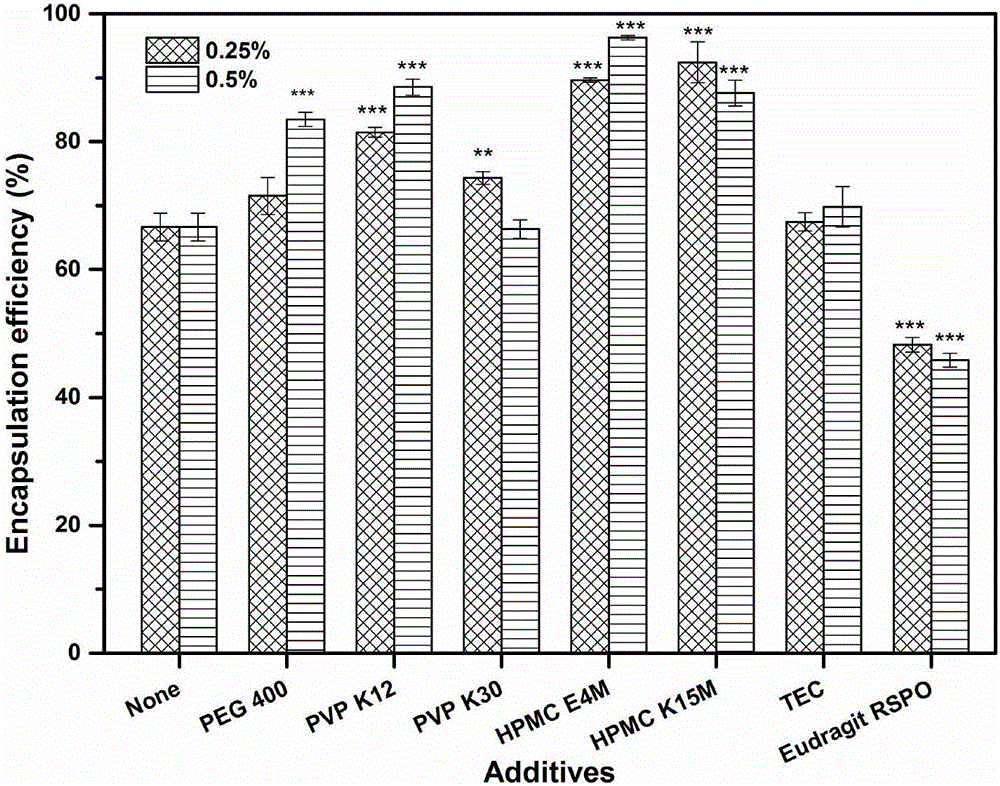
[0065] Take by weighing 21 mg of Huperzine A, dissolve in 200ml 80% v / v ethanol solution to obtain a drug solution, slowly add 10 g of framework material EC45 and additives of the type and dosage shown in Table 3 to the drug solution under the action of magnetic stirring until the skeleton material swells completely to form a uniform drug-containing polymer solution. The drug-containing polymer solution is fed into the UPPS high-speed rotating disc (9000rpm) through a peristaltic pump at an average speed of 7.8ml / min to form microdroplets, and then the solvent in the microdroplets is evaporated in the air field to further solidify into huperzine A skeleton particle. The encapsulation efficiency and release curve of the huperzine A matrix microparticles are carried out according to Example 2. The effects of various additives and their dosage on the encapsulation efficiency and drug release of huperzine A matrix particles are as follows: image 3 and Figure 4 shown. The res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com