Asymmetric synthesis for chiral huperzine A
A huperzine A, asymmetric technology, applied in the field of organic synthesis of natural products, can solve the problems of low yield, large amount of -8-phenylmenthol, lengthy reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
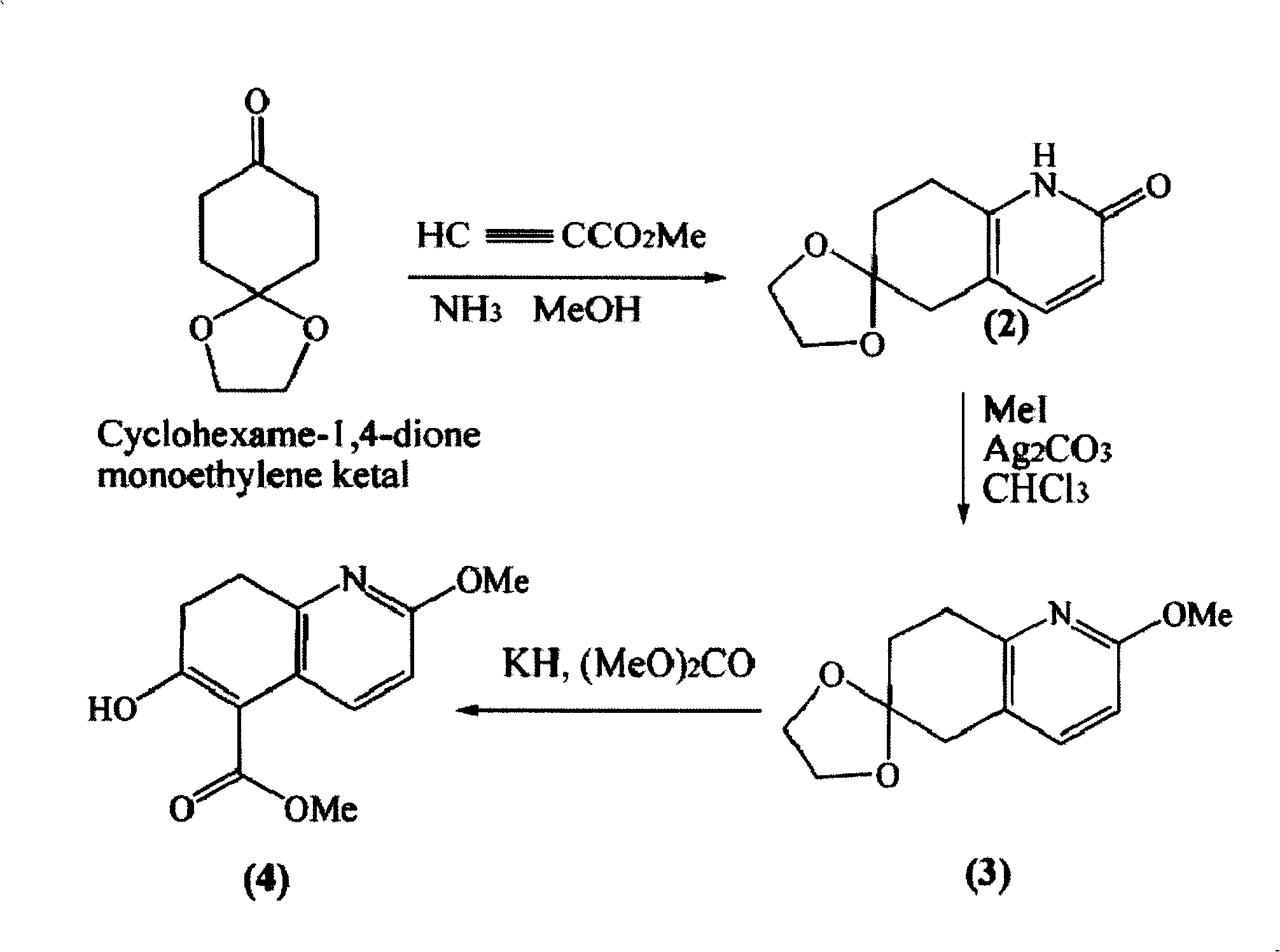
[0016] Example 1. Synthesis of intermediate β-ketoesters
[0017] Step 1, synthetic pyridone (2)
[0018] To a solution of 300 g (1.9 mol) of 1,4-dihydrospiro[4,5]-8-decanone dissolved in 6 L of ammonia-saturated methanol was added 320 g (3.8 mol) of methyl propiolate. The reaction mixture was heated to reflux at 100 °C for 10 h, and the internal pressure of the material reached a maximum of 200 psi. After cooling, the solvent was removed under reduced pressure to obtain 294 g of crude pyridone (2), a pale yellow solid. mp about 250°C.
[0019] Step 2, Synthesis of 7,8-dihydro-2-methoxyspiro[1,3-dioxolane-2,6(5H)-quinazoline (3)
[0020] At room temperature, under dark, crude product (2) and Ag 2 CO 3 867g (3.14mol) and 980ml (15.7mol) of methyl iodide dissolved in 20L of chloroform were mixed and stirred overnight. Filtration, concentration, and purification by dynamic axial compression chromatography (DAC) (40% ethyl acetate / hexanes) afforded ketal (3) 257 g (74%). m...
Embodiment 2
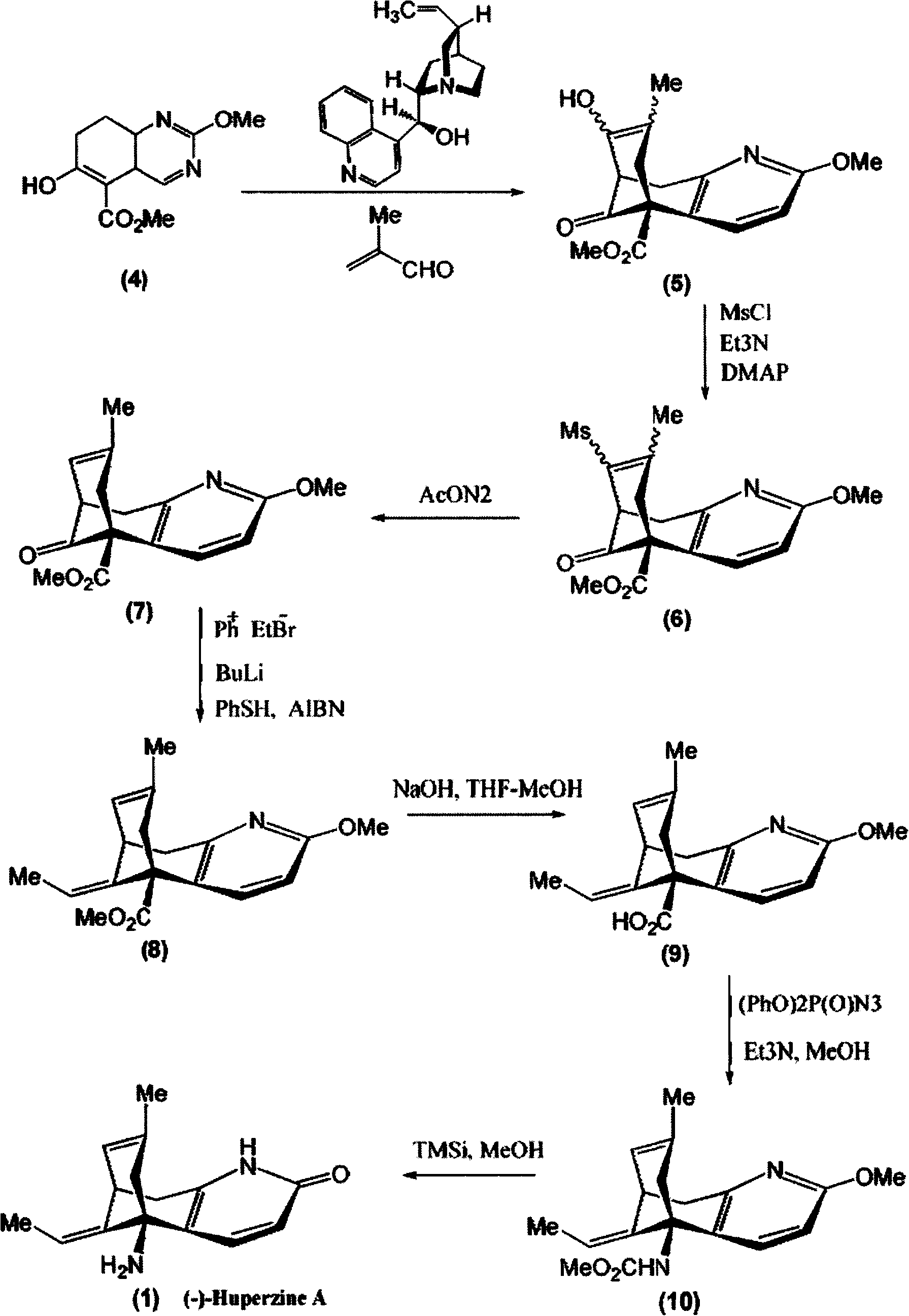
[0023] The synthesis of embodiment 2.(-)-huperzine A
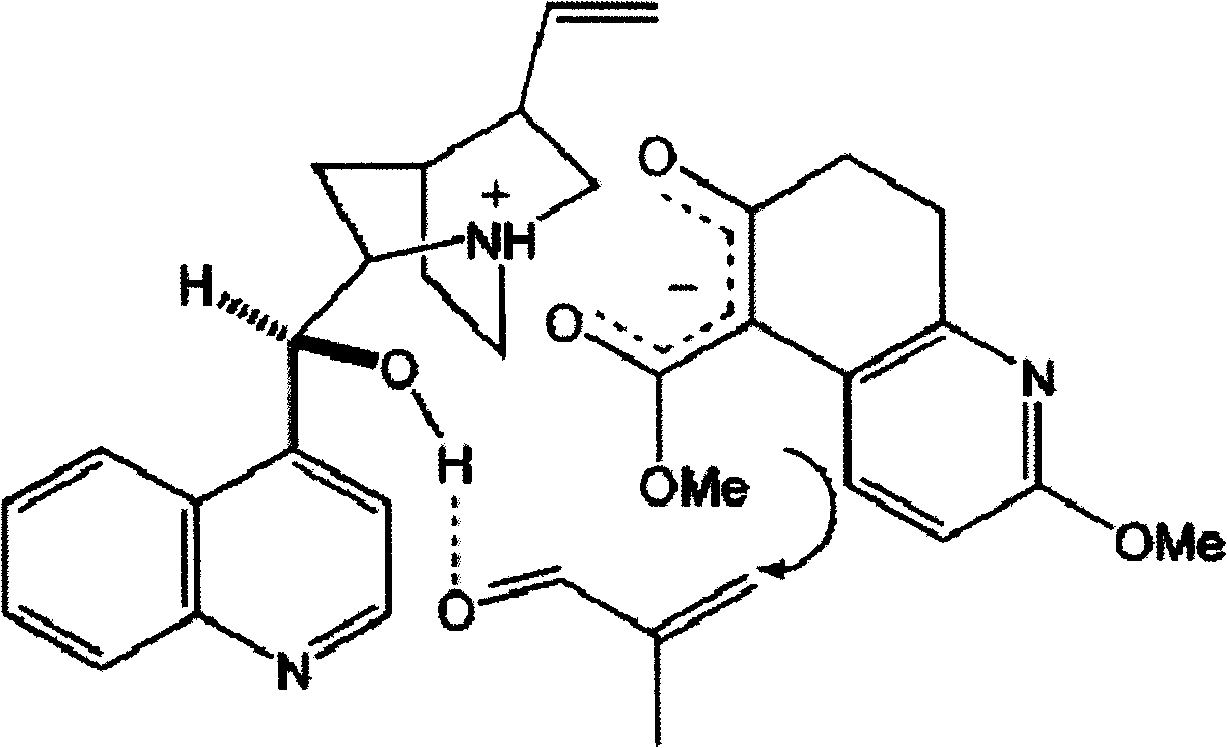
[0024] Step 1, Synthesis of methyl 7,8,9,10-tetrahydro-8-hydroxyl-2-methoxy-7-methyl-11-oxo-5,9-methylene ring aryl octapyridine-5 (6H)-Carboxylate (5)
[0025] At -10°C, under nitrogen protection, 88 μl (1.0 mmol) of methacrolein (1.0 mmol) and ( -)-cinchonidine 31.0 mg (0.10 mmol). The mixture was stirred at -10°C for 253h. After concentration under reduced pressure, the residue was purified by dynamic axial compression chromatography (DAC) (hexane / ethyl acetate, 3 / 2) to give (5) 14.6 mg (45%), a colorless oil (containing at least 3 diastereoisomers in a ratio of 10:7:1).
[0026] Step 2, synthesis of methyl 7,8,9,10-tetrahydro-8-methanesulfonyloxy-2-methoxy-7-methyl-11-oxo-5,9-methylenecycloaryl octane Pyridine-5(6hydro)-carboxylate (6)
[0027] At 0°C, under nitrogen protection, add 70 μl (0.50 mmol) of triethylamine (0.50 mmol) and 5.0 mg (41 μmol) of DMAP and 15 μl (0.19 mmol) of methanesulfonyl chloride (5) in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com