Reversible acetylcholinesterase inhibitor huperzine-A synthesis method
A technology for the synthesis of huperzine A, which is applied in the field of compound preparation, and can solve problems such as danger, the price of methyl propiolate is relatively expensive, and the inability to carry out large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
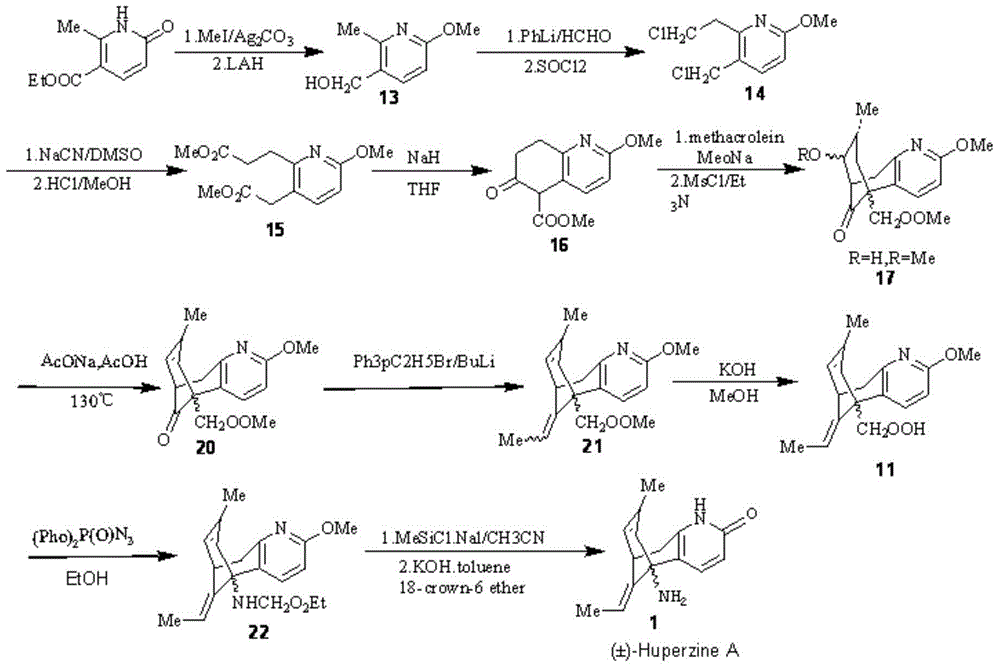
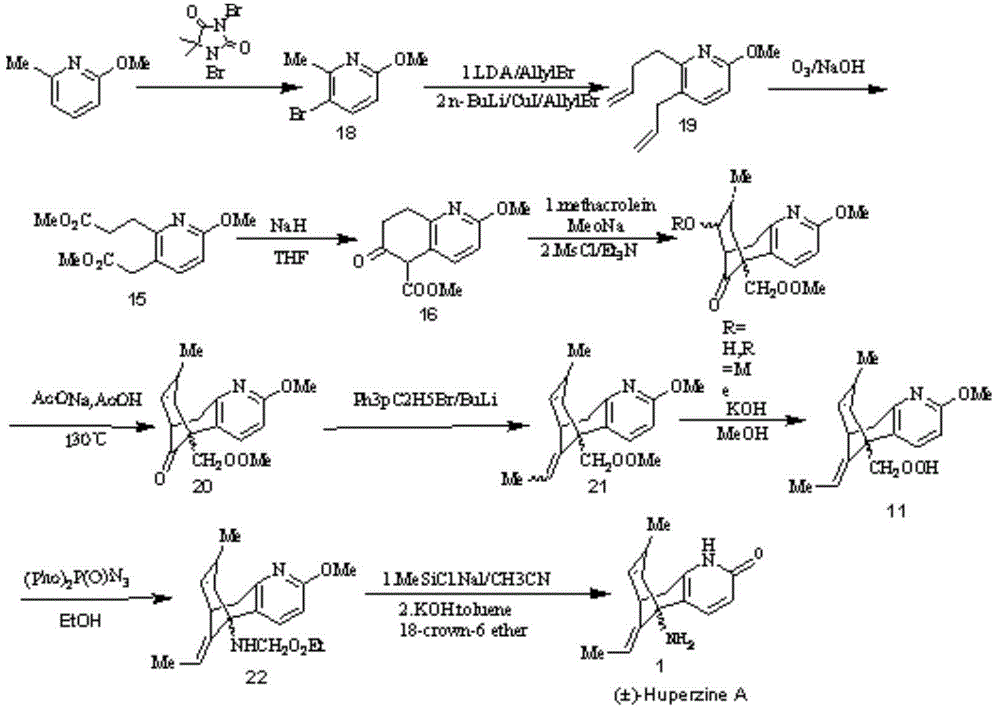
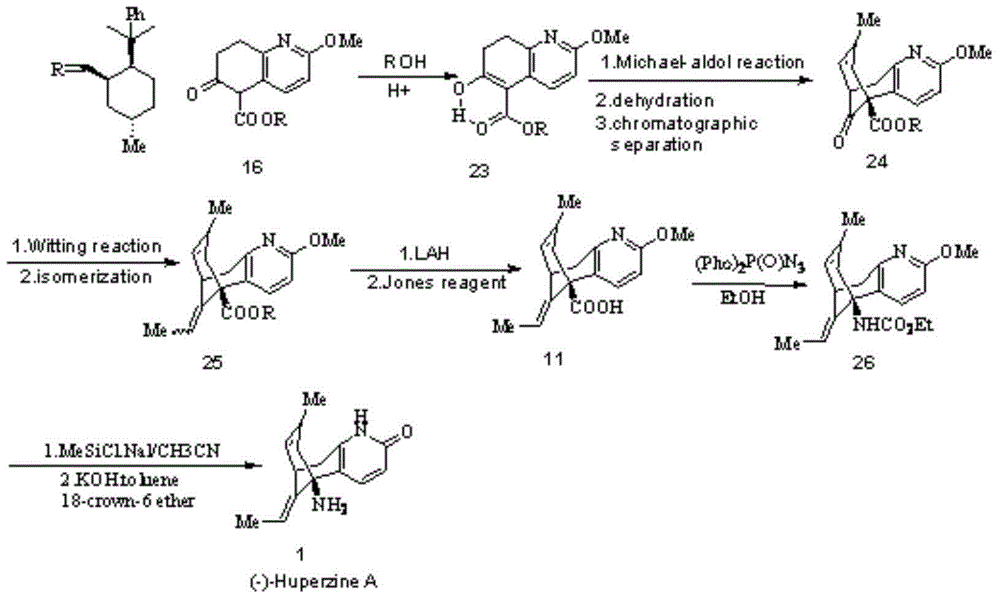
Image
Examples
Embodiment 1
[0160] (1) Preparation of compound 2 (2-cyanoethyl-1,4-cyclohexanedione-4-monoethylene glycol ketal)
[0161]
[0162] Add 650g of 1,4-cyclohexanedione monoethylene glycol ketal and 300g of tetrahydropyrrole into a 5L three-neck flask, add 2000mL of glacial acetic acid, and reflux for 4 hours. After the TLC reaction is complete, add 250g of acrylonitrile dropwise, and reflux for 12 hours. , TLC (thin layer chromatography) detects that the reaction is complete, and the glacial acetic acid is recovered under reduced pressure, and the oily matter obtained is dissolved with 3000mL methyl tert-butyl ether, washed with water, washed with saturated saline, dried in a dry bath, suction filtered, and concentrated to obtain 2-cyanoethyl -1,4-cyclohexanedione-4-monoethylene glycol ketal 850g, yield 98%, HPLC≥95.4%.
[0163] MS(ESI)m / z210.23[M+H] of Compound 2 + ;
[0164] Compound 2 1 H-MNR (300MHz, DMSO) δppm: δ2.08 (2H, t, J=6.5Hz, CH 2 ),δ2.15(2H,s,CH 2 ),δ2.27(2H,t,J=6.5Hz,CH...
Embodiment 2
[0222] (1) Preparation of compound 2 (2-cyanoethyl-1,4-cyclohexanedione-4-monoethylene glycol ketal)
[0223]
[0224] Add 400g of 1,4-cyclohexanedione monoethylene glycol ketal and 185g of tetrahydropyrrole into a 5L three-neck flask, add 2000mL of glacial acetic acid, and reflux for 5 hours. After the TLC reaction is complete, add 155g of acrylonitrile dropwise, and reflux for 20 hours. , TLC detected that the reaction was complete, recovered glacial acetic acid under reduced pressure, dissolved the obtained oil in 2000 mL of methyl tert-butyl ether, washed with water, washed with saturated saline, dried in a dry bath, filtered with suction, and concentrated to obtain 2-cyanoethyl-1,4-cyclo 520 g of hexanedione-4-monoethylene glycol ketal, yield 96%.
[0225] (2) Preparation of compound 3 (2-hydroxy-6-ethylene ketal-5,6,7,8-tetrahydroquinoline)
[0226]
[0227] Stir 200g of the above-mentioned 2-cyanoethyl-1,4-cyclohexanedione-4-monoethylene glycol ketal and 15g of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com