Preparation method of (-)-huperzine A
A technology for huperzine A and pharmaceutical preparations, applied in the field of splitting huperzine A
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
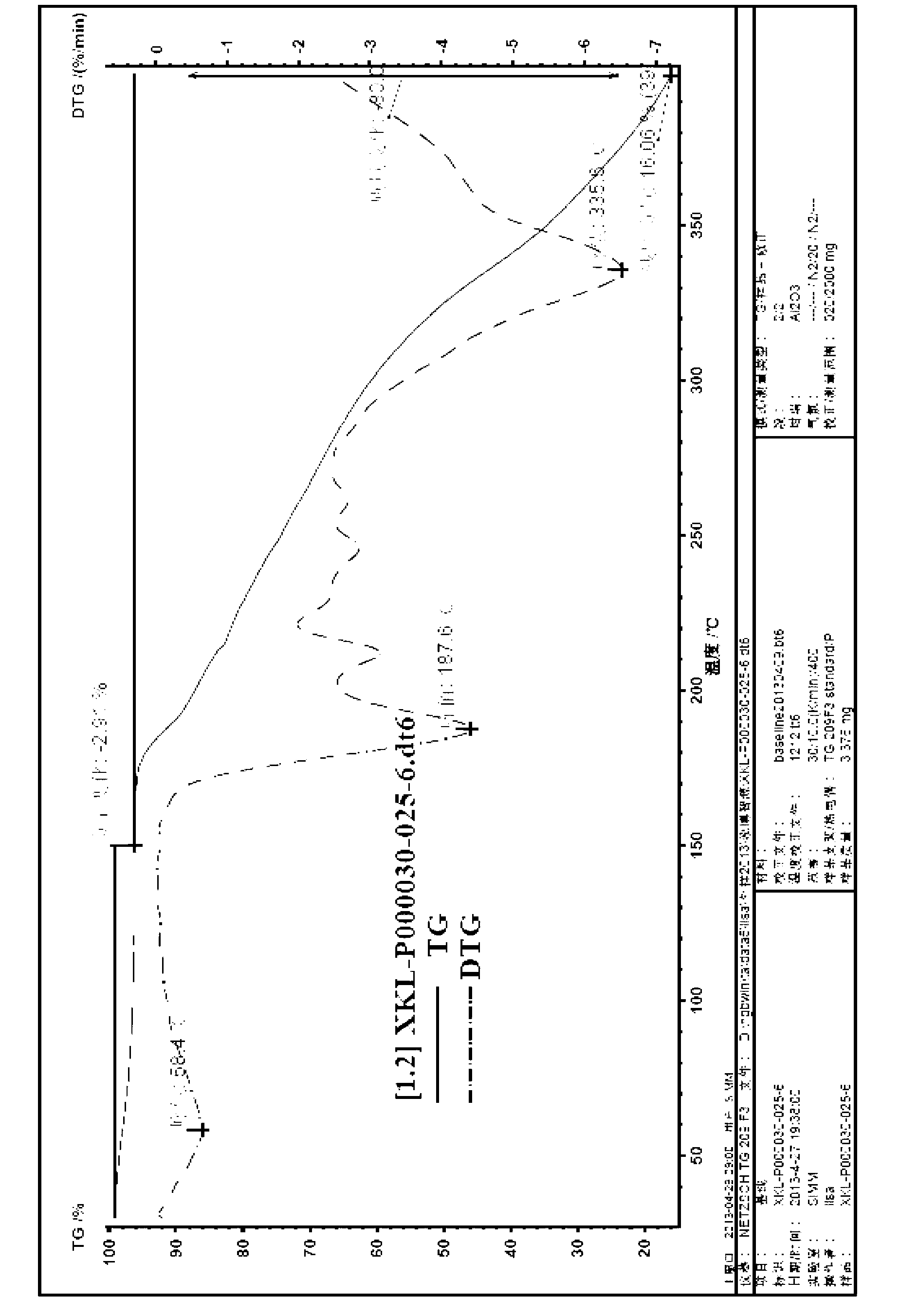
[0129] Preparation of (-)-huperzine A-D-dibenzoyl tartrate
[0130] 7.3g ( + )-huperzine A diastereomer mixture was suspended in a mixed solvent of acetone and water, 8.9g D-(-)-dibenzoyl tartaric acid was added at 20°C, stirred for 1 hour, filtered, and the solid was dissolved in absolute ethanol Recrystallization gave 8.5 g of (-)-huperzine A-D-dibenzoyl tartrate.
[0131] Yield: 82%; HPLC purity: 99% (310nm); optical purity: 99.5%. Melting point: 175-177°C
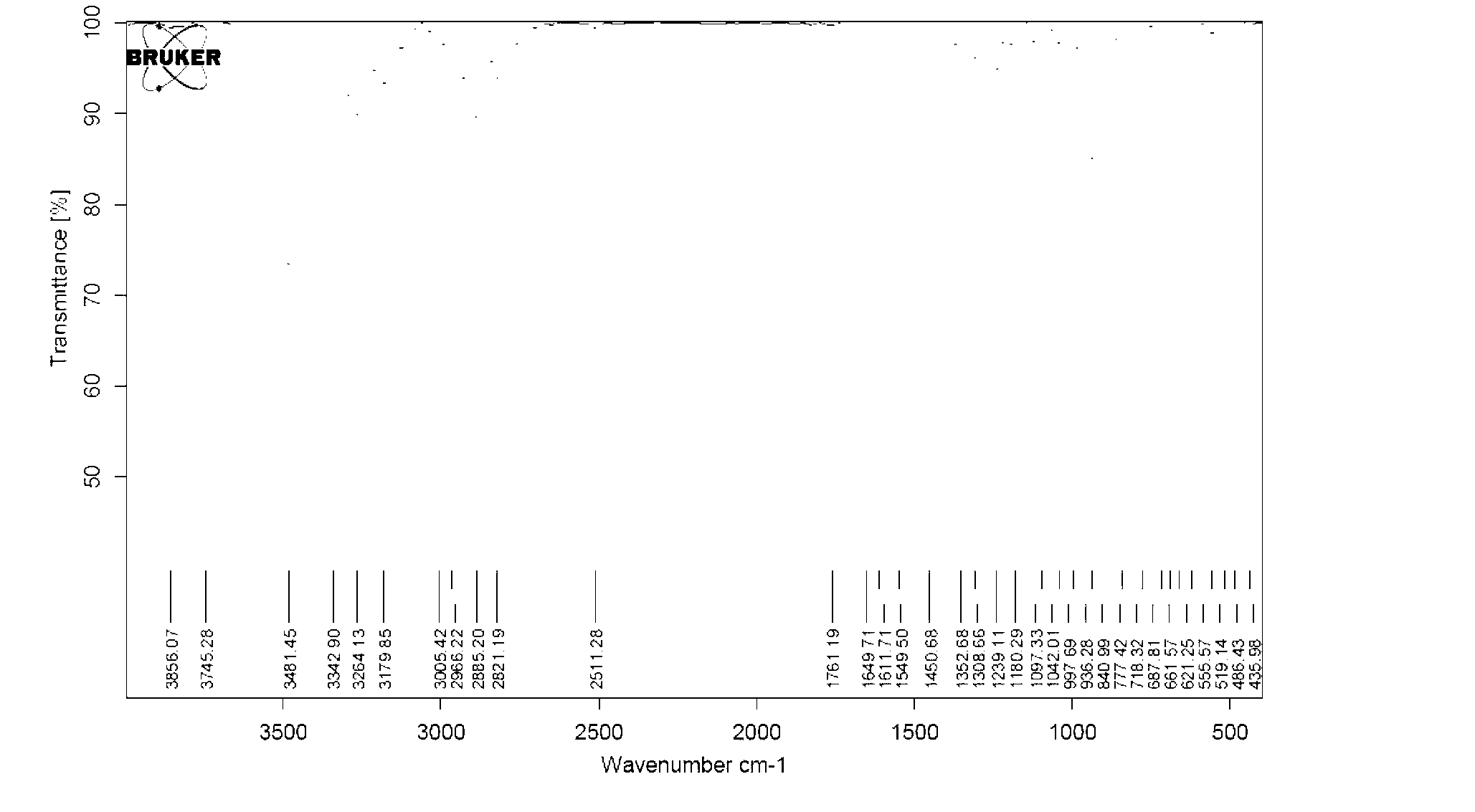
[0132] 1H NMR(400MHz,DMSO d6)δ7.96(d,J=7.6Hz,2H),7.79(d,J=9.6Hz,1H),7.61-7.65(m,1H),7.47-7.51(m,2H ),6.15(d,J=9.6Hz,1H),5.68(s,1H),5.41-5.46(m,2H),3.56(s,1H),2.63-2.68(m,1H),2.51-2.55( m,1H),2.11-2.31(m,2H),1.63(d,J=6.4Hz,3H),1.49(s,3H).
Embodiment 2
[0134] Preparation of (-)-Huperzine A
[0135] Add 8.5g (-)-huperzine A-D-dibenzoyl tartaric acid to 42.5ml water, adjust the pH to 9.0-9.3 with 40% sodium hydroxide, continue to stir for 1 hour, filter, and dissolve the solid in 10mL water Wash, recrystallize with 95% ethanol, and dry in vacuo to obtain 3.6 g of (-)-huperzine A.
[0136] Yield: 74%; HPLC purity: 99.6% (310nm); optical purity: 99.5%. Melting point: 217-219°C
[0137] 1 H NMR (400MHz, CDCl 3 )δ13.16(br,1H),7.92(d,J=9.6Hz,1H),6.43(d,J=9.6Hz,1H),5.47-5.52(m,1H),5.41-5.43(m,1H ),3.62(s,1H),2.88-2.93(m,1H),2.74-2.78(m,1H),2.12-2.19(m,2H),1.69(d,J=6.8Hz,3H),1.56( s,3H).
Embodiment 3
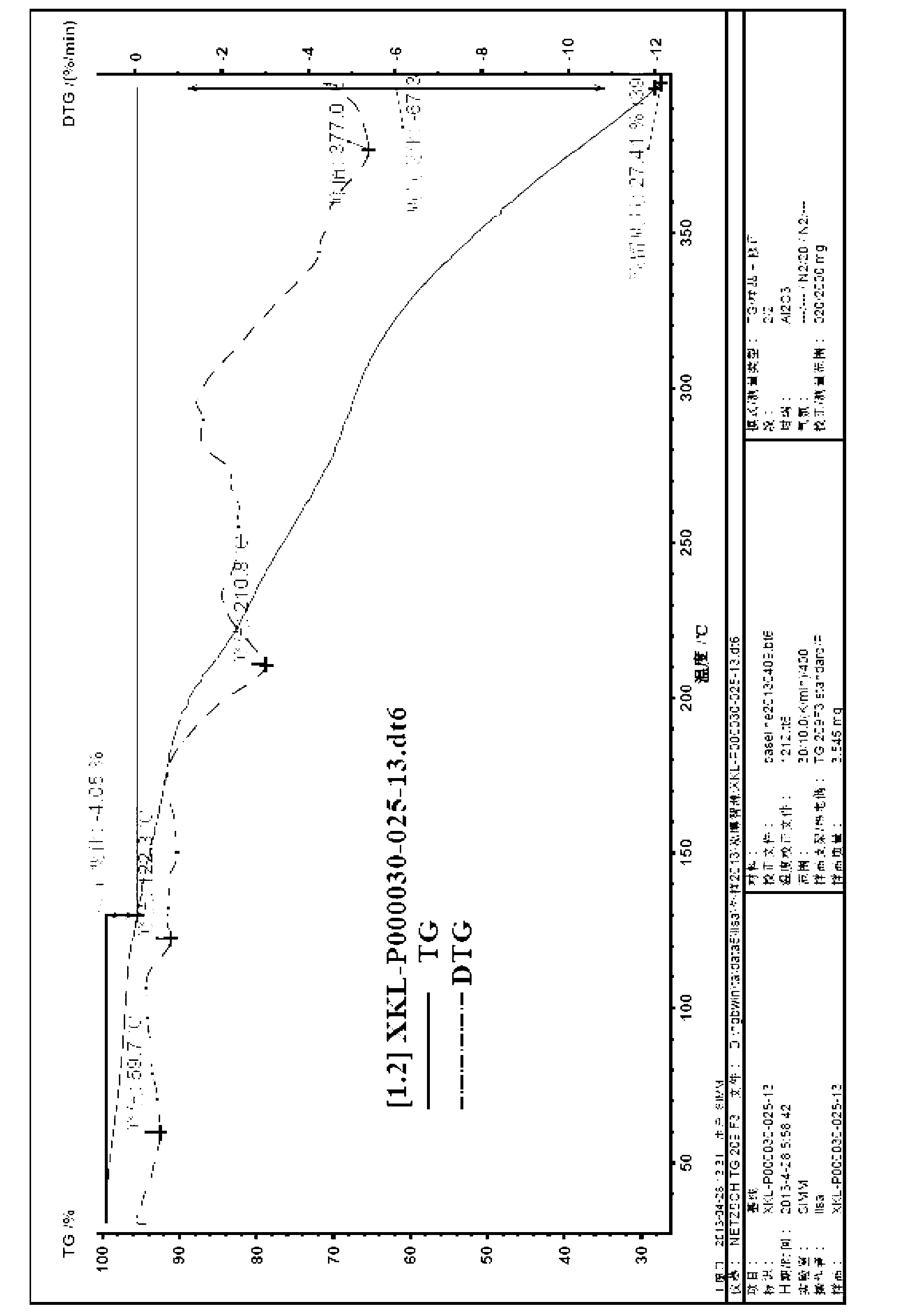
[0139] Preparation of (-)-Huperzine A-D-tartrate
[0140] Will 6g ( + )-Huperzine A diastereomer mixture was suspended in a mixed solvent of acetone and water, 3.1g (D)-tartaric acid was added at 20°C, stirred for 1 hour, filtered, and the solid was recrystallized from absolute ethanol to obtain (- )-huperzine A-D-tartrate 4g.
[0141] Yield: 51%; HPLC purity: 98% (310nm); optical purity: 95%. Melting point: 182-185°C
[0142] 1 H NMR (400MHz,D 2 O)δ7.87(d,J=9.6Hz,1H),6.63(d,J=9.6Hz,1H),5.63-5.64(m,1H),5.51-5.56(m,1H),4.51(s, 2H),3.88(s,1H),3.04-3.10(m,1H),2.79-2.84(m,1H),2.68-2.72(m,1H),2.53-2.57(m,1H),1.82(d, J=6.4Hz,3H),1.65(s,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com