Dosage forms for administering combinations of drugs
A composition and drug technology, applied in the directions of drug combinations, antipyretics, anti-inflammatory agents, etc., can solve the problems of delayed drug action, low therapeutic effect, weakened absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Preparation of pharmaceutical preparations
[0031] The pharmaceutical composition of the present invention includes tablets and capsules, which can be prepared according to standard methods in the art (see for example Remington's Pharmaceutical Sciences , 16 th ed., A Oslo editor, Easton, Pa. (1980)). Drugs and drug combinations will generally be prepared by blending with conventional excipients. Suitable carriers include, but are not limited to: water; saline solutions; alcohols; acacia; vegetable oils; benzyl alcohol; polyethylene glycol; gelatin; carbohydrates such as lactose, amylose, or starch; magnesium stearate; talc; silicon Acid; paraffin; aromatic oil; fatty acid ester; hydroxymethylcellulose; polyvinylpyrrolidone, etc. The pharmaceutical preparation can be sterilized, and if necessary, can also be mixed with adjuvants, such as: lubricants, preservatives, disintegrants; stabilizers; wetting agents; emulsifiers; salts; buffers; coloring agents; flavorin...
Embodiment 1
[0053] Example 1: Triptan and NSAID
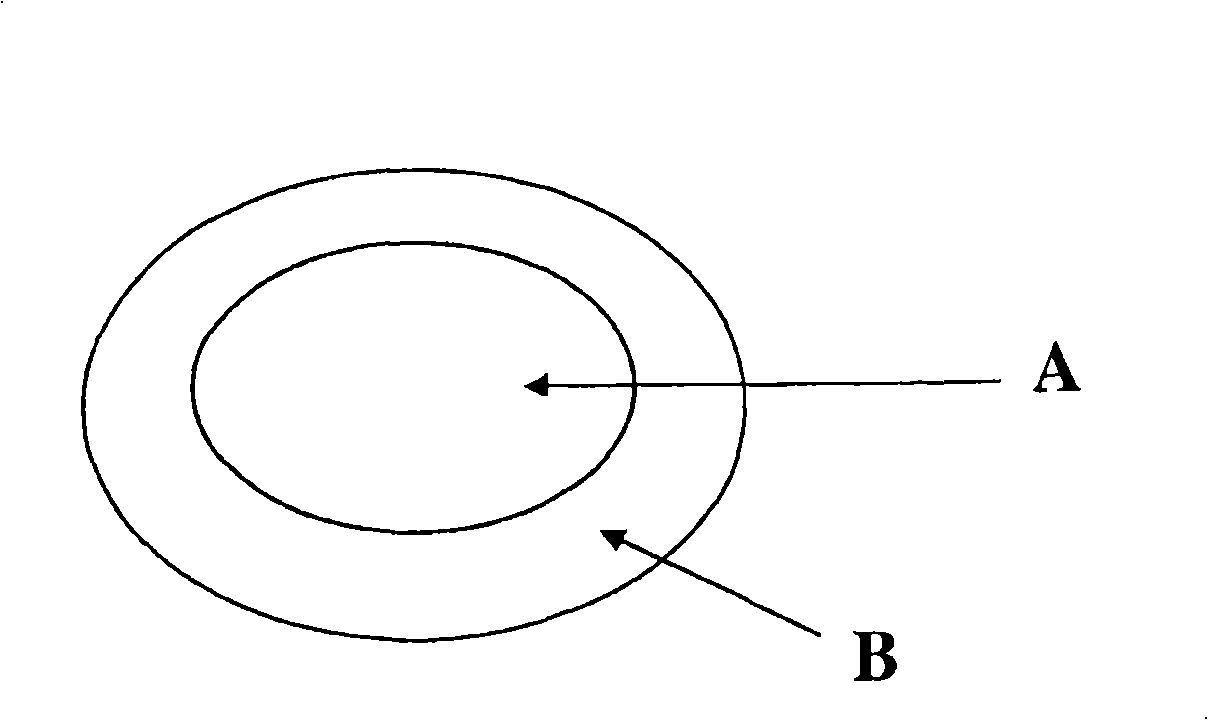
[0054] This example describes a press-coated and press-coated tablet consisting of sumatriptan succinate within a core and naproxen sodium surrounding the core. refer to figure 1 The tablet is shown.
[0055] Table 4: Composition of the core (40mg sumatriptan)
[0056] Element
Mg / piece
Intragranular components
Sumatriptan Succinate, USP 1
56.0
Lactose monohydrate, NF
56.0
Purified water, USP 2
QS
extragranular components
Anhydrous lactose, NF
112.0
Microcrystalline Cellulose, NF
26.2
Croscarmellose Sodium, NF
2.54
Magnesium Stearate, NF
1.27
total
254.0
[0057] 1 56.0mg sumatriptan succinate equivalent to 40mg sumatriptan
[0058] 2 Purified water, USP is removed during drying
[0059] Table 5: Composition of the core outer layer (500 mg naproxen sodium)
[0060] Element ...
Embodiment 2
[0066] Example 2: Opioid pain relievers and NSAIDs
[0067] This example describes a bilayer tablet consisting of sustained release hydrocodone and naproxen sodium. refer to figure 2 Schematic of the tablet or reference Figure 5 Tablets containing pellets are indicated.
[0068] Table 6: Composition of layer 1 (10 mg hydrocodone tartrate)
[0069] Element
Mg / piece
Intragranular components
Hydrocodone Tartrate, USP
10.0
Microcrystalline Cellulose, NF
37.5
Povidone, USP
15.0
Hydroxypropyl Methyl Cellulose (Methocel K4M)
45.0
Purified water, USP 1
QS
extragranular components
Microcrystalline Cellulose, NF
45.0
Magnesium Stearate, NF
1.5
total
154.0
[0070] 1 Purified water, USP is removed during drying
[0071] Table 7: Composition of layer 2 (400 mg naproxen sodium)
[0072] Element
Mg / piece
Int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com