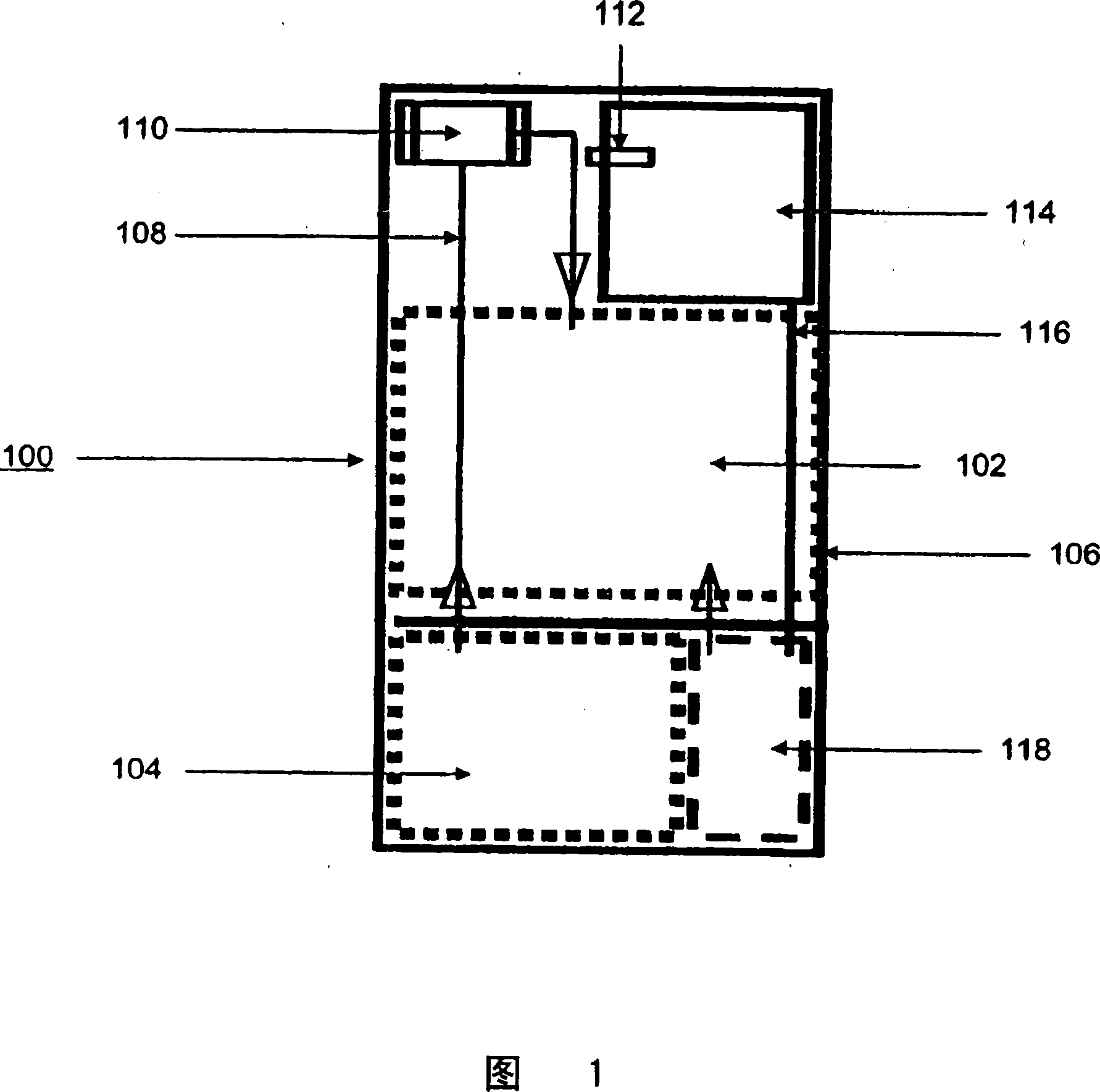
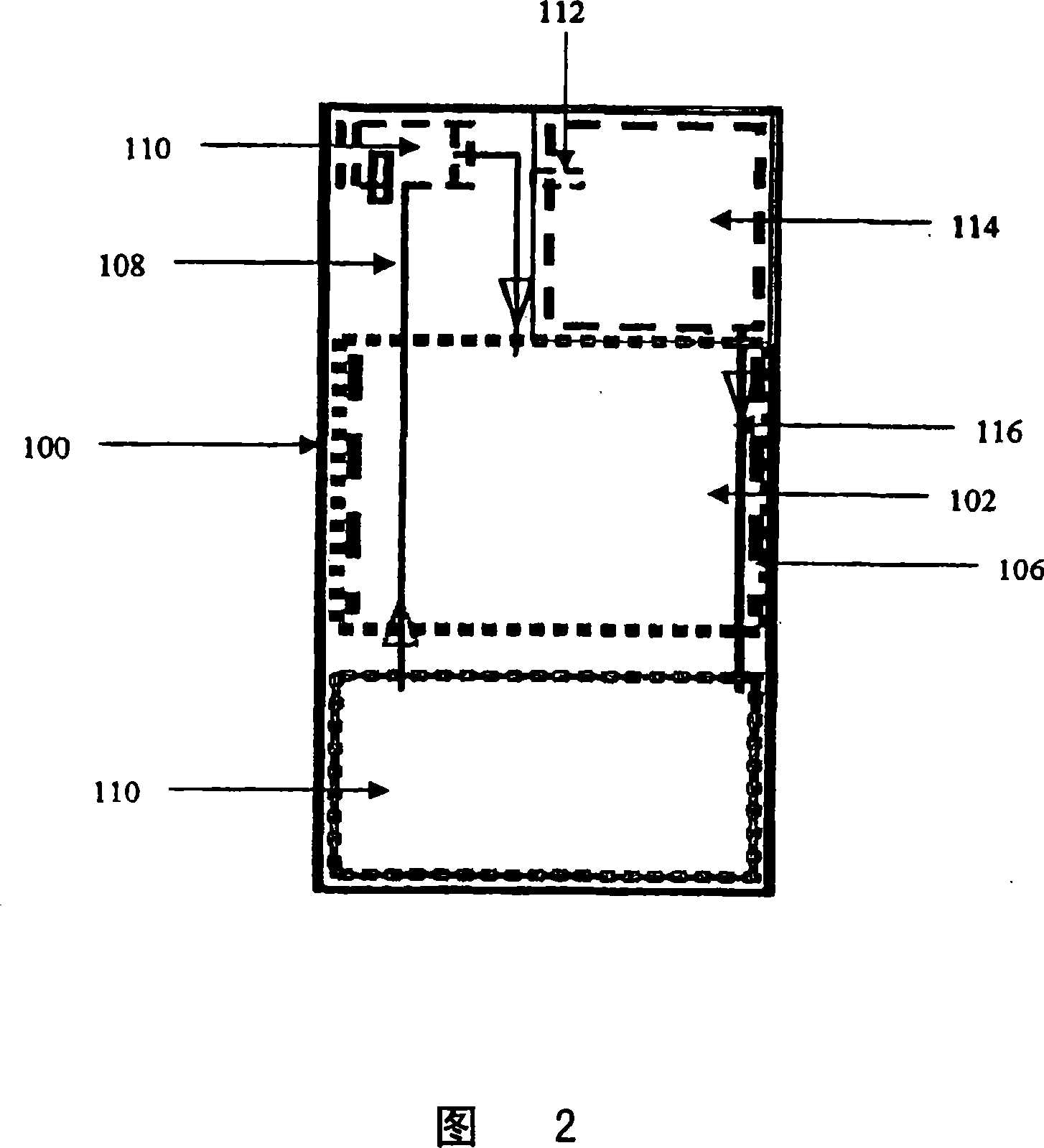
Systems and methods for hydrogen generation from solid hydrides
A technology of borohydride and hydrogen, applied in the field of systems and methods for generating hydrogen from solid hydrides, capable of solving problems such as limiting hydrogen storage density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
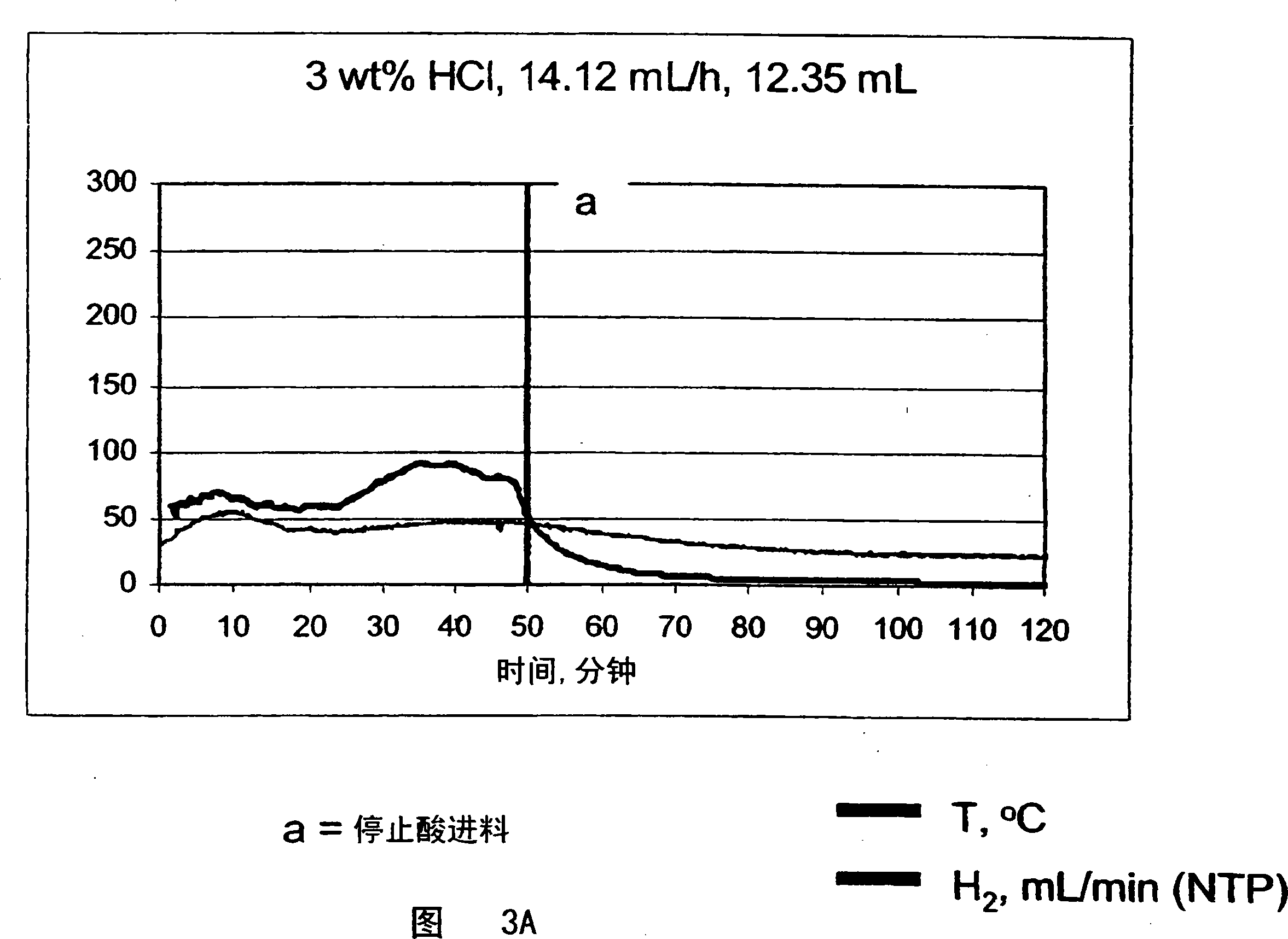
[0056] Measurement of system kinetics and H in a semi-batch reactor system with solid granular sodium borohydride loaded in a 250 mL Pyrex reactor 2 flow rate. Hydrochloric acid (HCl) was supplied with a syringe pump at the indicated flow rates and durations (Table 1). The reaction temperature was monitored with an internal thermocouple. The hydrogen was cooled to room temperature via a water / ice bath and any moisture was removed from the gas stream by passing it through a bed of silica gel. Measure the dry H with an online mass flow meter 2 flow rate. After each run was completed, the sodium borohydride conversion was analyzed using NMR of the reaction mixture.
[0057] The hydrogen production reaction can be stopped at various conversion levels by stopping the acid solution feed. This provides an efficient mechanism to control hydrogen production. The maximum temperature and maximum hydrogen flow rate of the system can be adjusted using the acid flow rate, as shown in ...
Embodiment 2
[0062] Using the procedure described in Example 1, kinetic hydrogen production rates were measured after periodic start-stop cycles at an acid solution feed rate of 10 wt-% HCl 10 mL / h. The acid flow was started and stopped repeatedly, the reactor was cooled to ambient temperature between stop / start cycles, and the rate of hydrogen production was measured, as shown in Figure 6. As the reaction proceeds, the solid sodium borohydride is converted to a mixture of borate compounds. Droplet diffusion of the acid solution through these products to unreacted sodium borohydride resulted in a somewhat reduced third-cycle reaction rate, but the start and stop kinetics remained fast.
Embodiment 3
[0064] According to one test, to 5 g of solid NaBH in a sealed container 4 1 wt% hydrochloric acid aqueous solution was added dropwise. Hydrogen evolution from this reaction was monitored with a mass flow meter. Figure 7 graphically illustrates the hydrogen flow rate when acidified water is added. Under the experimental conditions, the amount of hydrogen evolved is proportional to the amount of acid added, and the overall hydrogen yield corresponds to approximately 100% conversion of borohydride to hydrogen. The system response after hydrogen addition is also very fast, less than about 5s. Add to NaBH 4 The amount of water is NaBH 4 About 5 times the molar amount.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com