Sulfur tolerant alumina catalyst support
A kind of alumina and sulfur-resistant technology, applied in the direction of alumina/aluminum hydroxide, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem of not showing hydrothermal stability , to enhance the activity of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2 and comparative example C1-C4
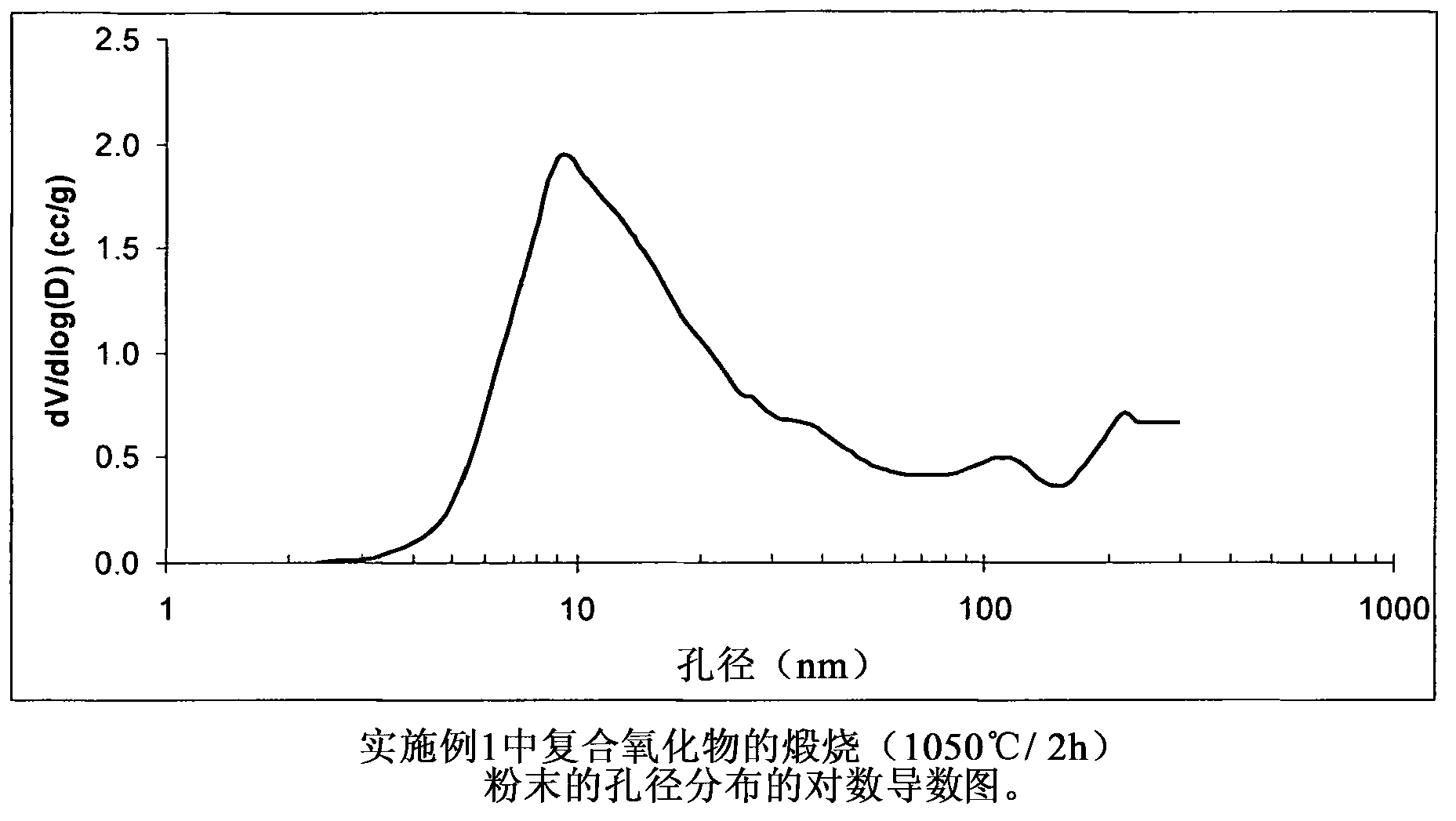
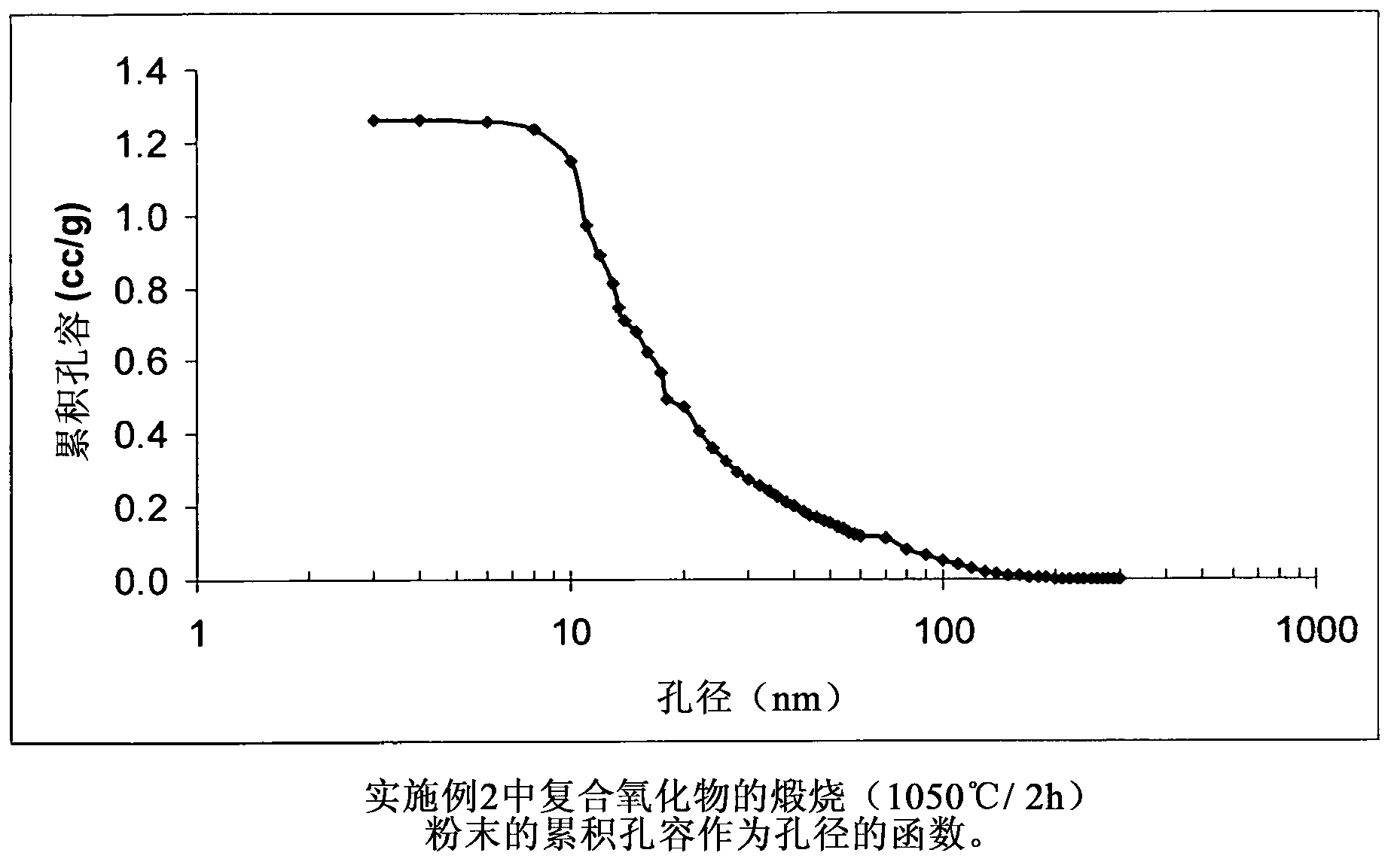
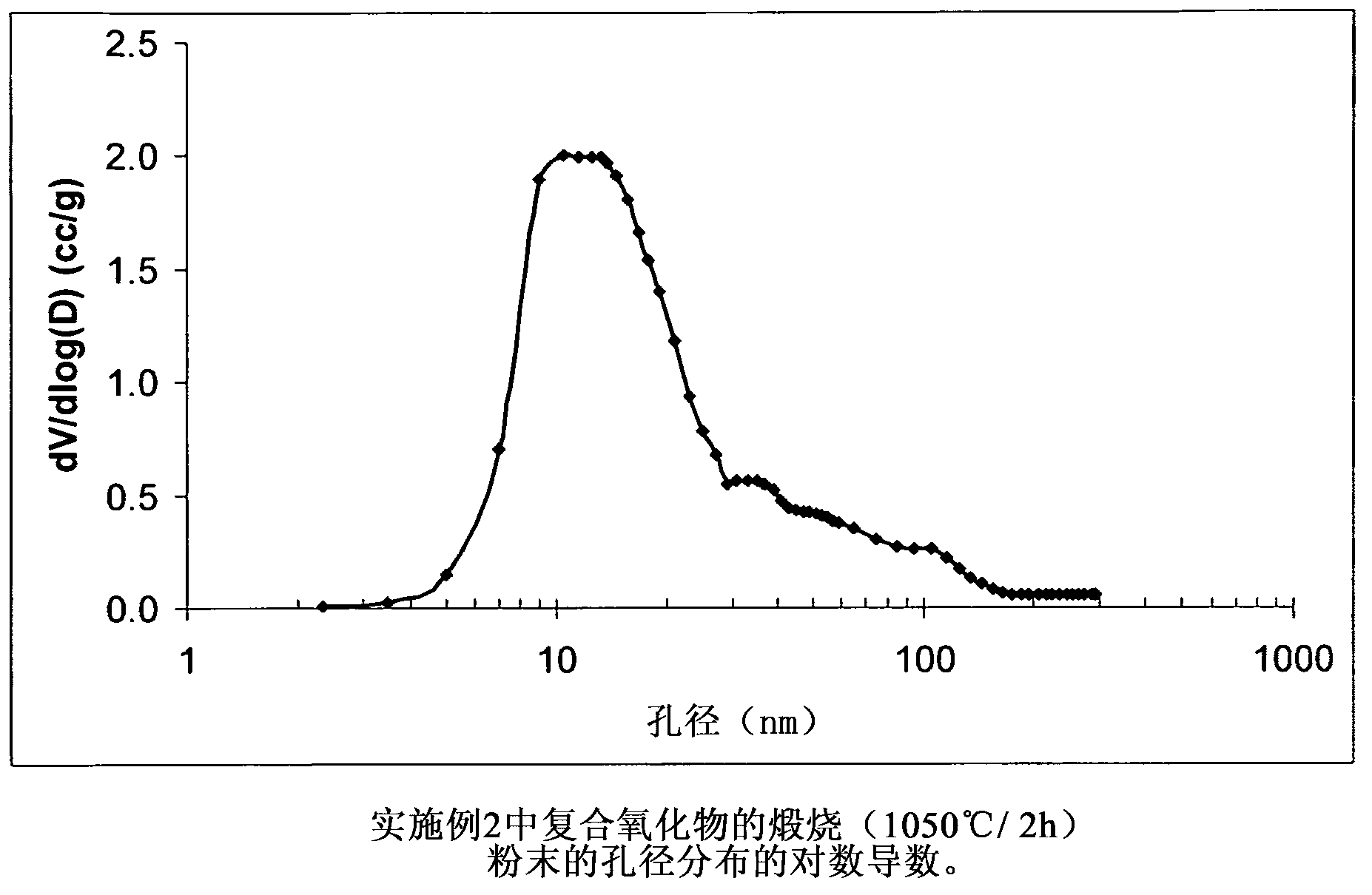
[0080] The composite oxide of Example 1 contains (based on 100 pbw of the composite oxide) 80 pbw of Al 2 o 3 and 20pbw of SiO 2 , the composite oxide was prepared by using aluminum sulfate, sodium aluminate and sodium silicate in the following manner. Solution A is an aqueous solution of aluminum sulfate, the concentration of which is aluminum oxide Al 2 o 3 Expressed as 8.31% by weight. Solution B is an aqueous solution of sodium aluminate, the concentration of which is aluminum oxide Al 2 o 3 Expressed as 24.86% by weight. Solution C is an aqueous solution of sodium silicate, and its concentration uses silicon dioxide SiO 2 Expressed as 29.21% by weight. 424 g of deionized water were added to the 1 L reactor. The reactor contents were heated at 65° C. and this temperature was maintained throughout the experiments unless otherwise stated hereinafter. 6.02 g of Solution A were introduced into the reactor under agitation over a period of 5 minutes. The reactor conte...
Embodiment 3
[0111] The composite oxide of Example 3 contains (based on 100 pbw of composite oxide) 65 pbw of Al 2 o 3 , 20pbw of SiO 2 and 15pbw of ZrO 2 , the composite oxide was prepared in the same manner as in Example 2, except that zirconium nitrate (concentration 21.3%, density 1.306) was mixed with aluminum sulfate solution before precipitation. The spray-dried powder exhibited a surface area of 459 m 2 / g. The spray-dried powder was calcined at 900°C for 2 hours and at 1050°C for 2 hours. Table VII below shows the surface area, pore volume results. Measure the specific surface area ("SA", in square meters per gram ("m 2 / g"), pore volume (in cubic centimeters / gram ("cm 3 / g")) and average pore size (expressed in nanometers ("nm")), and for both calcination temperatures (expressed in degrees Celsius ("°C") and time (expressed in hours ("h")) The results are recorded in Table VII below.
[0112] Table VII
[0113]
[0114] Figure 4 The logarithmic derivative of the ...
Embodiment 4
[0120] The composite oxide of Example 4 contains (based on 100 pbw of composite oxide) 69 pbw of Al 2 o 3 , 16pbw of SiO 2 and 13pbw of TiO 2 , the composite oxide was prepared in the same manner as in Example 2, except that: prior to precipitation, titanium orthosulfate (concentration 9.34%, density 1.376) was mixed with aluminum sulfate solution. The spray-dried powder exhibited a surface area of 488 m 2 / g. The spray-dried powder was calcined at 750°C for 2 hours and at 900°C for 2 hours. The powder sample that had been calcined at 750°C / 2h was then calcined at 1100°C for 5 hours, at 1200°C for 5 hours, and at 1050°C for 2 hours. The results determined for the above-mentioned different calcination conditions are shown in Table IX below: surface area (in square meters per gram ("m 2 / g"), pore volume (in cubic centimeters / gram ("cm 3 / g")) and average pore diameter (expressed in nanometers ("nm")).
[0121] Table IX
[0122]
[0123] Figure 7 The logarithmi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com