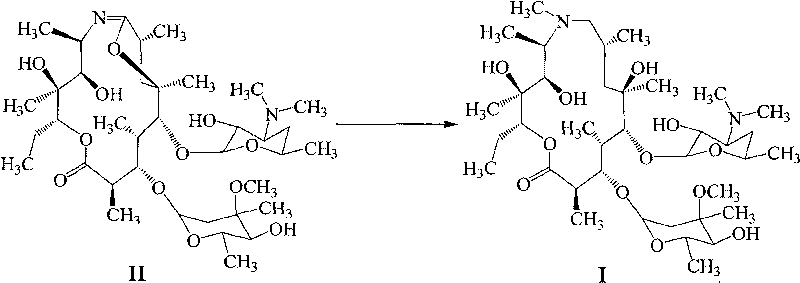
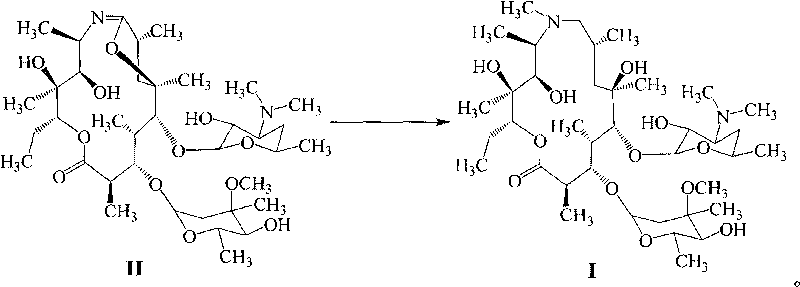
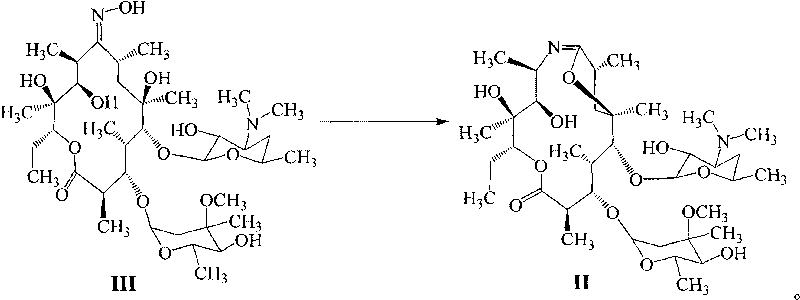
Method for preparing azithromycin and method for preparing intermediate of azithromycin
A technology of azithromycin and erythromycin, applied in the field of preparation of azithromycin, can solve the problems of high dosage of reducing agent sodium borohydride, low yield of azithromycin, high price of azithromycin, etc., and achieve moderate dosage of reducing agent, lower cost and yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 erythromycin A9-oxime
[0037] Add 40g of hydroxylamine hydrochloride and 120g of methanol into a 500ml reaction flask, add 40g of triethylamine dropwise (about 40 minutes), raise the temperature to 40°C and stir for three hours, add a buffer solution with a molar ratio of triethylamine and acetic acid of 1:2 to control When the pH value is 5-8, add 100 g of erythromycin thiocyanate (the content of erythromycin thiocyanate A is 80%), react for 5 hours, heat up and reflux for 18 hours, cool down to 20°C, and drip 100 g of water within 30 minutes. -200ml, stirred for 30 minutes and filtered to obtain oxime salt, washed with water. Add dichloromethane and water, adjust the pH value to 9-11 with 10% aqueous sodium hydroxide solution, and let stand to separate layers. The upper aqueous layer was extracted once more with dichloromethane, and the dichloromethane layers were combined. Add water, evaporate dichloromethane, cool down in ice bath,...
Embodiment 2
[0038] The preparation of embodiment 2 erythromycin A9-oxime
[0039] Add 40 g of hydroxylamine hydrochloride, 120 g of methanol into a 500 ml reaction flask, add 40 g of diethylamine dropwise (about 40 minutes), raise the temperature to 40°C and stir for three hours, add a buffer with a molar ratio of diethylamine:acetic acid of 1:2 Control the pH value of the solution at 5-8, add 100 g of erythromycin thiocyanate (the content of erythromycin thiocyanate A is 80%), react for five hours, heat up and reflux for 18 hours, cool down to 20°C, and drip water within 30 minutes 100-200ml, stirred for 30 minutes, filtered to obtain oxime salt, washed with water. Add dichloromethane and water, adjust the pH value to 9-11 with 10% aqueous sodium hydroxide solution, and let stand to separate layers. The upper aqueous layer was extracted once more with dichloromethane, and the dichloromethane layers were combined. Add water, evaporate dichloromethane, cool down in ice bath, filter, wash...
Embodiment 3
[0040] Embodiment 3 erythromycin A6, the preparation of 9-imine ether
[0041] Add 80g of erythromycin A9-oxime and 30g of alkaline agent sodium bicarbonate into a 1000ml reaction flask, add 100mL of water and 100mL of dichloromethane each, lower the temperature below 25°C, add 50g of methanesulfonyl chloride dropwise, and finish the dropwise addition within half an hour. Insulation reaction, standing for stratification, extracting the organic layer with water twice, combining the water layers, adjusting the pH value to above 11 with 10% sodium hydroxide solution and stirring for one hour, cooling in an ice bath, suction filtration, washing with water to obtain the product, and drying , about 74.4g, the yield is 93%, the HPLC purity content is 93% after detection, and the moisture content is less than 5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com